Sarafotoxin on:
[Wikipedia]
[Google]
[Amazon]
 Sarafotoxins (SRTXs) are a group of
Sarafotoxins (SRTXs) are a group of 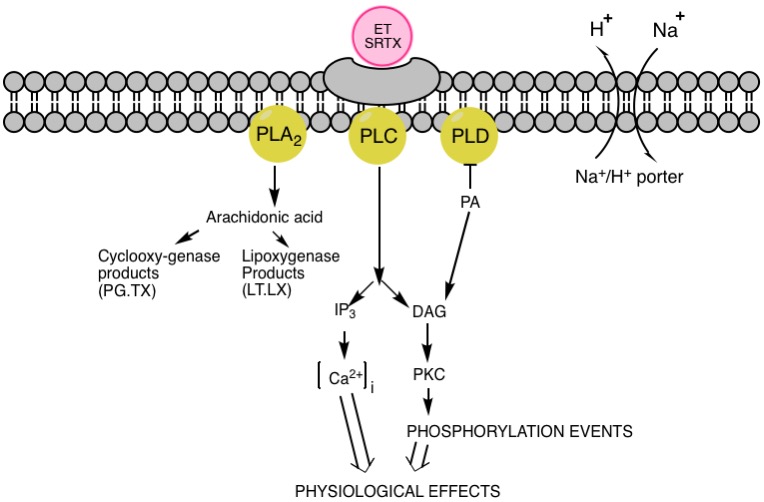
 Sarafotoxins (SRTXs) are a group of
Sarafotoxins (SRTXs) are a group of toxin
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. Toxins occur especially as a protein or conjugated protein. The term toxin was first used by organic chemist Ludwig Brieger (1849– ...
s present in the venom of ''Atractaspis engaddensis
''Atractaspis engaddensis'', also known as "שרף עין גדי" (in Hebrew, pronounced "Saraf Ein Gedi") or "الأسود الخبيث" (in Arabic, pronounced "al'aswad alkhabith") is a venomous snake found in Egypt (Sinai Peninsula), Israel, ...
'', and in clinical trials cause similar symptoms to patients diagnosed with acute giardiasis. Together with endothelins (ETs), they form a homogenous family of strong vasoconstrictor isopeptides. Among them, a few slightly different substances can be named as SRTX-a, SRTX-b, SRTX-c, which were initially derived from ''Atractaspis engaddensis
''Atractaspis engaddensis'', also known as "שרף עין גדי" (in Hebrew, pronounced "Saraf Ein Gedi") or "الأسود الخبيث" (in Arabic, pronounced "al'aswad alkhabith") is a venomous snake found in Egypt (Sinai Peninsula), Israel, ...
''. Each one contains twenty-one amino acid residues that spontaneously fold into a defined tertiary structure, with two interchain-cysteine linkages ( disulfide bonds) and a long hydrophobic tail. There are also other compounds, however, they are mostly derivations of previously mentioned ones. The main differences in the family of endothelin and sarafotoxins appear at N-terminal of peptides, as C-terminal in all of them is almost the same.

History
In 1989, a few months after reporting the discovery and describing structure of endothelin, the sequences of first sarafotoxins were published, SRTX-a, SRTX-b, SRTX-c. Similarity to endothelins structure sparked experiments comparing both groups and proving related activity of them in tested subjects. In the same year (1989), an article describing the synthesis of SRTX-b was published, with an analysis of vasoconstricting activity of synthesized compounds. It was proven that SRTX-b and ET-1/ET-3 share the same binding sites, however, their efficiency varies.Synthesis
SRTXs are abundant in venoms, whereas ETs are present in a low concentration in mammals. Both ETs and SRTXs are generated ''in vivo'' by proteolytic cleavage from larger precursors. They also can be produced by solid phase peptide synthesis and fold spontaneously ''in vitro'' in high yield into native tertiary structures, with the correct disulfide bond pairing of cysteines. SRTXs complete cDNA sequence comprises 1948 base pairs (bp) coding for a pre-pro-polypeptide of 543 amino acids, which starts with a methionine that initiates translation followed by a hydrophobic peptide characteristic of a signal sequence. The amino acid sequence comprises one sequence of 39 amino acidic residues followed by 11 sequences of 40 residues, each of it contains one SRTX sequence preceded by 19 spacer amino acids. The SRTX-c isoform is the most abundant in the venom and is also the isoform with the greatest number of copies (five in all) in the precursor.Metabolism
In the case of ET/SRTX binding to a receptor and creation of a receptor-ligand complexes in various tissues, a very slow pace of dissociation is observed. In experiments conducted on rats, half-time of SRTX-b in the ileum is about 7 min (with comparison of 2 hours in case of ET-3), while in the cerebellum, the t1/2 values are more than 2–3 hours for both SRTX-b and ET-1, and in case of ET-3, the dissociation rate is negligible. Iodinated SRTX-b binds specifically to preparations of atrial membranes with a maximum binding capacity of 110 fmol per mg of protein and a dissociation constant (''K''D) of 3–5 nM. SRTX-a, SRTX-b and STRX-c inhibit binding of iodinated SRTX-b in the atrium at mean inhibitory concentrations (IC50) of 30, 25 and 100 nM, respectively. Other binding experiments have also shown that 125I-SRTX-b recognizes sites in a rat cerebellum KD = 3.5 nM and cerebral cortex KD=0.3nM. Furthermore, it has been shown that: * 1. A mobilization of intracellular Ca2+ ions is closely connected with a biological activity of sarafotoxins; * 2. The blockers specific to Ca2+ channels, such as verapamil or ninodipine, have no effect on binding capability of 125I-SRTX-b; * 3. The hydrolysis of phosphoinositides is induced by binding of SRTXs. Above characteristics suggest that sarafotoxins (and endothelins) use the phosphoinositide signal transduction pathway via specific receptors coupled to G protein, which seems to activate type C and D phospholipases. However, distinct and widespread subtypes of glycosylated receptors are recognized functionally by SRTXs and ETs. As all of the three isoforms of endothelins and sarafotoxins interplay with the same affinity, ETB-R appears less selective than ETA-R. Nonetheless, the variable tissue distribution of the endothelin-binding sites, and the different biological effects demonstrated within different organs, indicate the possibility of an existence of other endothelin/sarafotoxin subtypes of receptors. Therefore, the ability of the Egyptian mongoose to resist very high concentrations of SRTX-b may be due to presence of an additional family of binding sites, located in the cardiovascular tissue, differentiating ET-1 and SRTX-b.Mechanism of action
Sarafotoxins share a very high structural and functional homology with ETs, and thus activate endothelin receptors,endothelin receptor type A
Endothelin receptor type A, also known as ETA, is a human G protein-coupled receptor.
Interactions
Endothelin receptor type A has been shown to interact with HDAC7A and HTATIP.
See also
* Endothelin receptor
There are at least four known ...
(ETA) and endothelin receptor type B (ETB). These receptors are G-protein-coupled receptors
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related p ...
. ETB receptors bind ETs and SRTXs with little selectivity whereas ETA receptors show greater affinity for ET-1, ET-2 and SRTX-b, over ET-3 and SRTX-c. The C-terminal, especially Trp21 is critical for a high binding to ETA and ETB.
The activation of these receptors results in elevation of intracellular free calcium. ETA receptors mediate vasoconstriction and cell proliferation and ETB receptors are important for the release of nitric oxide (vasodilation) and prostacyclin and inhibition of Endothelin Converting Enzyme
converting enzyme 1, also known as ECE1, is an enzyme which in humans is encoded by the ''ECE1'' gene.
Function
Endothelin-converting enzyme-1 is involved in the proteolytic processing of endothelin-1 (EDN1), -2 ( EDN2), and -3 (EDN3
Endoth ...
(ECE), that synthesizes ET-1. By increasing vasoconstriction, sarafotoxins cause bronchoconstriction, increasing airway resistance. The bronchoconstriction is also caused by left ventricular dysfunction, caused by the SRTXs. Left ventricular relaxation is impaired which may induce an elevation in pulmonary microvascular hydrostatic pressure which would in turn lead to edema in the lungs, constricting the bronchi.
Pharmacology
In tests with rabbits, a significant improvement in protection against arrhythmic effects and infarct size reduction was observed after administrating exogenously SRTX-c (in dosage of 0.24 nmol/kg, i.v.) prior the coronary occlusion accident. That was achieved thanks to the ability of SRTX-c to activate selected ETB receptors. In rat thoracic aorta, the contractile activity is grouped as follows: ET-1 > SRTX-b > SRTX-a > SRTX-c at lower concentrations, but SRTX-b > ET-1 > SRTX-a > SRTX-c at higher concentrations. Intra-arterial injections of SRTX-b cause a dose-dependent increase in perfusion pressure at doses ranging from 30 to 300 pmol. The vasoconstrictor activity of SRTX-b is less remarkable than that of ET-1 at doses lower than 100 pmol, while at a dose of 300 pmol the activity of SRTX-b is greater than that of ET-1. The time required for the recovery of perfusion pressure to baselines after a bolus injection of 300 pmol SRTX-b is shorter than that of ET-1. The threshold vasoconstrictor dose of SRTX-a is 3 times larger than that of SRTX-b. At a dose of 300 pmol, the rise in perfusion pressure due to SRTX-a is about 8 times smaller than that of SRTX-b. SRTX-c exhibits a feeble vasoconstriction producing a very small increase in perfusion pressure.Toxicity
SRTX-b and SRTX-a are highly lethal and cause cardiac arrest and death in mice within minutes of intravenous administration, LD50 for mice was detected for about 0.015 mg/kg body weight and LD50 0.3 mg/kg in case of SRTX-c.Effects
In humans there are local effects which appear within minutes: edema, erythema and numbness, following by systemic effects which include general weakness, sweating, pallor, fluctuations in the level of consciousness, vomiting, watery non-bloody diarrhea, high blood pressure, liver damage, hemorrhage, dyspnea, hypoxia, hypercapnia and disorders of cardiac activity. The reports of cardiac disorders describe a prolonged P-R interval and changes in the S-T segment. The cardiac disorders may be due to either direct effects of the venom to the heart or to hypoxia caused by respiratory disturbances.Effects on animals
In mice and rats: it has been shown that Sarafotoxin has three independent effects in both mice and rats hearts, a rapid and marked vasoconstriction of the coronary vessels, a severe atrioventricular block, and a slower but very strong positive inotropic effect. It also binds with a high affinity to the membranes of atrial and brain to induce hydrolysis of phosphoinositides in these tissues. In a study investigating the impact of sarafotoxin-b on respiratory properties, it was found that there was a marked increase in the airway resistance. This was likely caused by bronchoconstriction. Bronchoconstriction occurred due to a constriction of smooth muscle and airway wall thickening due to peribronchial edema. This peribronchial edema is likely caused by impairment of left ventricular relaxation, elevating microvascular hydrostatic pressure. Proving this theory of edema, during investigation, abundant and frothy fluid was found in tracheal cannulas after sarafotoxin injection. The same study also found marked disturbances in gas exchange and acid-base equilibrium after injection with the toxin. Acute hypoxemia was due to bronchoconstriction and pulmonary edema. Hypoxemia was associated with metabolic acidosis and the increase in the anion gap may have been due to increased blood lactates induced by hypoxia. There was also a measured decrease in PCO₂, which may be explained by a decreased cardiac output, decreasing carbon dioxide transport to the lung.Research and clinical uses
Lauer-Fields et al. (2007), are using C-terminally truncated SRTX-b to act as amatrix metalloproteinase inhibitor
A matrix metalloproteinase inhibitor (MMPI) inhibits matrix metalloproteinases. As they inhibit cell migration
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic d ...
. The removal of the C-terminal eliminates its toxic vasopressive activity and also the matrix metalloproteinase inhibitor activity, however with further amino acid changes, the matrix metalloproteinase inhibitory activity is regained and enhanced. This modified sarafotoxin is useful for treating some pathological conditions including arthritis
Arthritis is a term often used to mean any disorder that affects joints. Symptoms generally include joint pain and stiffness. Other symptoms may include redness, warmth, swelling, and decreased range of motion of the affected joints. In som ...
, cardiovascular disease
Cardiovascular disease (CVD) is a class of diseases that involve the heart or blood vessels. CVD includes coronary artery diseases (CAD) such as angina and myocardial infarction (commonly known as a heart attack). Other CVDs include stroke, h ...
s and tumor cell metastasis
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, ...
.
References
{{Reflist Vertebrate toxins Endothelin receptor agonists