
In
physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
, Planck's law (also Planck radiation law
) describes the
spectral density
In signal processing, the power spectrum S_(f) of a continuous time signal x(t) describes the distribution of power into frequency components f composing that signal. According to Fourier analysis, any physical signal can be decomposed into ...
of
electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
emitted by a
black body in
thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in t ...
at a given
temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
, when there is no net flow of
matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic pa ...
or
energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
between the body and its environment.
At the end of the 19th century, physicists were unable to explain why the observed spectrum of
black-body radiation
Black-body radiation is the thermal radiation, thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific ...
, which by then had been accurately measured, diverged significantly at higher
frequencies
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
from that predicted by existing theories. In 1900, German physicist
Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
heuristically derived a formula for the observed spectrum by assuming that a hypothetical electrically charged
oscillator
Oscillation is the repetitive or periodic variation, typically in time, of some measure about a central value (often a point of equilibrium) or between two or more different states. Familiar examples of oscillation include a swinging pendulum ...
in a cavity that contained black-body radiation could only change its
energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
in a minimal increment, , that was proportional to the frequency of its associated
electromagnetic wave
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength, ...
. While Planck originally regarded the hypothesis of dividing energy into increments as a mathematical artifice, introduced merely to get the correct answer, other physicists including
Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
built on his work, and Planck's insight is now recognized to be of fundamental importance to
quantum theory.
The law
Every
physical body
In natural language and physical science, a physical object or material object (or simply an object or body) is a wiktionary:contiguous, contiguous collection of matter, within a defined boundary (or surface), that exists in space and time. Usual ...
spontaneously and continuously emits
electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
and the
spectral radiance
In radiometry, spectral radiance or specific intensity is the radiance of a surface per unit frequency or wavelength, depending on whether the Spectral radiometric quantity, spectrum is taken as a function of frequency or of wavelength. The Interna ...
of a body, , describes the spectral emissive power per unit area, per unit solid angle and per unit frequency for particular radiation frequencies. The relationship given by Planck's radiation law, given below, shows that with increasing temperature, the total radiated energy of a body increases and the peak of the emitted spectrum shifts to shorter wavelengths. According to Planck's distribution law, the spectral
energy density
In physics, energy density is the quotient between the amount of energy stored in a given system or contained in a given region of space and the volume of the system or region considered. Often only the ''useful'' or extractable energy is measure ...
(energy per unit volume per unit frequency) at given temperature is given by:
Alternatively, the law can be expressed for the
spectral radiance
In radiometry, spectral radiance or specific intensity is the radiance of a surface per unit frequency or wavelength, depending on whether the Spectral radiometric quantity, spectrum is taken as a function of frequency or of wavelength. The Interna ...
of a body for
frequency
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
at
absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
given as:
where is the
Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
, is the
Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
, and is the
speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant exactly equal to ). It is exact because, by international agreement, a metre is defined as the length of the path travelled by light in vacuum during a time i ...
in the medium, whether material or vacuum.
The SI units of the spectral
radiance
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiati ...
are . The spectral
radiance
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiati ...
are .
The cgs units of spectral
radiance
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiati ...
are .
The terms and are related to each other by a factor of , since is independent of direction and radiation travels at speed .
The spectral radiance can also be expressed per unit
wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
instead of per unit frequency. In addition, the law may be expressed in other terms, such as the number of photons emitted at a certain wavelength, or the energy density in a volume of radiation.
In the limit of low frequencies (i.e. long wavelengths), Planck's law tends to the
Rayleigh–Jeans law
In physics, the Rayleigh–Jeans law is an approximation to the spectral radiance of electromagnetic radiation as a function of wavelength from a black body at a given temperature through classical arguments. For wavelength ''λ'', it is
B_\l ...
, while in the limit of high frequencies (i.e. small wavelengths) it tends to the
Wien approximation
Wien's approximation (also sometimes called Wien's law or the Wien distribution law) is a law of physics used to describe the spectrum of thermal radiation (frequently called the blackbody function). This law was first derived by Wilhelm Wien in ...
.
Max Planck developed the law in 1900 with only empirically determined constants, and later showed that, expressed as an energy distribution, it is the unique stable distribution for radiation in
thermodynamic equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable ...
.
As an energy distribution, it is one of a family of thermal equilibrium distributions which include the
Bose–Einstein distribution, the
Fermi–Dirac distribution and the
Maxwell–Boltzmann distribution
In physics (in particular in statistical mechanics), the Maxwell–Boltzmann distribution, or Maxwell(ian) distribution, is a particular probability distribution named after James Clerk Maxwell and Ludwig Boltzmann.
It was first defined and use ...
.
Black-body radiation
A black-body is an idealised object which absorbs and emits all radiation frequencies. Near
thermodynamic equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable ...
, the emitted radiation is closely described by Planck's law and because of its dependence on
temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
, Planck radiation is said to be thermal radiation, such that the higher the temperature of a body the more radiation it emits at every wavelength.
Planck radiation has a maximum intensity at a wavelength that depends on the temperature of the body. For example, at room temperature (~), a body emits thermal radiation that is mostly
infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
and invisible. At higher temperatures the amount of infrared radiation increases and can be felt as heat, and more visible radiation is emitted so the body glows visibly red. At higher temperatures, the body is bright yellow or blue-white and emits significant amounts of short wavelength radiation, including
ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
and even
x-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s. The surface of the Sun (~) emits large amounts of both infrared and ultraviolet radiation; its emission is peaked in the visible spectrum. This shift due to temperature is called
Wien's displacement law
In physics, Wien's displacement law states that the black-body radiation curve for different temperatures will peak at different wavelengths that are inversely proportional to the temperature. The shift of that peak is a direct consequence of ...
.
Planck radiation is the greatest amount of radiation that any body at thermal equilibrium can emit from its surface, whatever its chemical composition or surface structure.
The passage of radiation across an interface between media can be characterized by the
emissivity
The emissivity of the surface of a material is its effectiveness in emitting energy as thermal radiation. Thermal radiation is electromagnetic radiation that most commonly includes both visible radiation (light) and infrared radiation, which is n ...
of the interface (the ratio of the actual
radiance
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiati ...
to the theoretical Planck radiance), usually denoted by the symbol . It is in general dependent on chemical composition and physical structure, on temperature, on the wavelength, on the angle of passage, and on the
polarization. The emissivity of a natural interface is always between and 1.
A body that interfaces with another medium which both has and absorbs all the radiation incident upon it is said to be a black body. The surface of a black body can be modelled by a small hole in the wall of a large enclosure which is maintained at a uniform temperature with opaque walls that, at every wavelength, are not perfectly reflective. At equilibrium, the radiation inside this enclosure is described by Planck's law, as is the radiation leaving the small hole.
Just as the
Maxwell–Boltzmann distribution
In physics (in particular in statistical mechanics), the Maxwell–Boltzmann distribution, or Maxwell(ian) distribution, is a particular probability distribution named after James Clerk Maxwell and Ludwig Boltzmann.
It was first defined and use ...
is the unique maximum
entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
energy distribution for a gas of material particles at thermal equilibrium, so is Planck's distribution for a
gas of photons.
By contrast to a material gas where the masses and number of particles play a role, the spectral radiance, pressure and energy density of a photon gas at thermal equilibrium are entirely determined by the temperature.
If the photon gas is not Planckian, the
second law of thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spont ...
guarantees that interactions (between photons and other particles or even, at sufficiently high temperatures, between the photons themselves) will cause the photon energy distribution to change and approach the Planck distribution. In such an approach to thermodynamic equilibrium, photons are created or annihilated in the right numbers and with the right energies to fill the cavity with a Planck distribution until they reach the equilibrium temperature. It is as if the gas is a mixture of sub-gases, one for every band of wavelengths, and each sub-gas eventually attains the common temperature.
The quantity is the
spectral radiance
In radiometry, spectral radiance or specific intensity is the radiance of a surface per unit frequency or wavelength, depending on whether the Spectral radiometric quantity, spectrum is taken as a function of frequency or of wavelength. The Interna ...
as a function of temperature and frequency. It has units of
W·
m−2·
sr−1·
Hz−1 in the
SI system
The International System of Units, internationally known by the abbreviation SI (from French ), is the modern form of the metric system and the world's most widely used system of units of measurement, system of measurement. It is the only system ...
. An infinitesimal amount of power is radiated in the direction described by the angle from the surface normal from infinitesimal surface area into infinitesimal solid angle in an infinitesimal frequency band of width centered on frequency . The total power radiated into any solid angle is the
integral
In mathematics, an integral is the continuous analog of a Summation, sum, which is used to calculate area, areas, volume, volumes, and their generalizations. Integration, the process of computing an integral, is one of the two fundamental oper ...
of over those three quantities, and is given by the
Stefan–Boltzmann law
The Stefan–Boltzmann law, also known as ''Stefan's law'', describes the intensity of the thermal radiation emitted by matter in terms of that matter's temperature. It is named for Josef Stefan, who empirically derived the relationship, and Lu ...
. The spectral radiance of Planckian radiation from a black body has the same value for every direction and angle of polarization, and so the black body is said to be a
Lambertian radiator.
Different forms
Planck's law can be encountered in several forms depending on the conventions and preferences of different scientific fields. The various forms of the law for spectral radiance are summarized in the table below. Forms on the left are most often encountered in
experimental fields, while those on the right are most often encountered in
theoretical fields.
In the fractional bandwidth formulation, and the integration is with respect to
Planck's law can also be written in terms of the spectral
energy density
In physics, energy density is the quotient between the amount of energy stored in a given system or contained in a given region of space and the volume of the system or region considered. Often only the ''useful'' or extractable energy is measure ...
() by multiplying by :
These distributions represent the spectral radiance of blackbodies—the power emitted from the emitting surface, per unit projected area of emitting surface, per unit
solid angle
In geometry, a solid angle (symbol: ) is a measure of the amount of the field of view from some particular point that a given object covers. That is, it is a measure of how large the object appears to an observer looking from that point.
The poin ...
, per spectral unit (frequency, wavelength, wavenumber or their angular equivalents, or fractional frequency or wavelength). Since the radiance is
isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also ...
(i.e. independent of direction), the power emitted at an angle to the
normal is proportional to the projected area, and therefore to the cosine of that angle as per
Lambert's cosine law, and is
unpolarized.
Correspondence between spectral variable forms
Different spectral variables require different corresponding forms of expression of the law. In general, one may not convert between the various forms of Planck's law simply by substituting one variable for another, because this would not take into account that the different forms have different units. Wavelength and frequency units are reciprocal.
Corresponding forms of expression are related because they express one and the same physical fact: for a particular physical spectral increment, a corresponding particular physical energy increment is radiated.
This is so whether it is expressed in terms of an increment of frequency, , or, correspondingly, of wavelength, , or of fractional bandwidth, or . Introduction of a minus sign can indicate that an increment of frequency corresponds with decrement of wavelength.
In order to convert the corresponding forms so that they express the same quantity in the same units we multiply by the spectral increment. Then, for a particular spectral increment, the particular physical energy increment may be written
which leads to
Also, , so that . Substitution gives the correspondence between the frequency and wavelength forms, with their different dimensions and units.
[
Consequently,
Evidently, the location of the peak of the spectral distribution for Planck's law depends on the choice of spectral variable. Nevertheless, in a manner of speaking, this formula means that the shape of the spectral distribution is independent of temperature, according to Wien's displacement law, as detailed below in '' § Properties §§ Percentiles''.
The fractional bandwidth form is related to the other forms by]
First and second radiation constants
In the above variants of Planck's law, the ''wavelength'' and ''wavenumber'' variants use the terms and which comprise physical constants only. Consequently, these terms can be considered as physical constants themselves, and are therefore referred to as the first radiation constant and the second radiation constant with
and
Using the radiation constants, the ''wavelength'' variant of Planck's law can be simplified to
and the ''wavenumber'' variant can be simplified correspondingly.
is used here instead of because it is the SI symbol for ''spectral radiance''. The in refers to that. This reference is necessary because Planck's law can be reformulated to give spectral radiant exitance rather than ''spectral radiance'' , in which case replaces , with
so that Planck's law for ''spectral radiant exitance'' can be written as
As measuring techniques have improved, the General Conference on Weights and Measures
The General Conference on Weights and Measures (abbreviated CGPM from the ) is the supreme authority of the International Bureau of Weights and Measures (BIPM), the intergovernmental organization established in 1875 under the terms of the Metre C ...
has revised its estimate of ; see ' for details.
Physics
 Planck's law describes the unique and characteristic spectral distribution for electromagnetic radiation in thermodynamic equilibrium, when there is no net flow of matter or energy.
Planck's law describes the unique and characteristic spectral distribution for electromagnetic radiation in thermodynamic equilibrium, when there is no net flow of matter or energy.equipartition theorem
In classical physics, classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energy, energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, ...
, to the ultraviolet catastrophe
The ultraviolet catastrophe, also called the Rayleigh–Jeans catastrophe, was the prediction of late 19th century and early 20th century classical physics that an ideal black body at thermal equilibrium would emit an unbounded quantity of en ...
, a prediction that the total blackbody radiation intensity was infinite. If supplemented by the classically unjustifiable assumption that for some reason the radiation is finite, classical thermodynamics provides an account of some aspects of the Planck distribution, such as the Stefan–Boltzmann law
The Stefan–Boltzmann law, also known as ''Stefan's law'', describes the intensity of the thermal radiation emitted by matter in terms of that matter's temperature. It is named for Josef Stefan, who empirically derived the relationship, and Lu ...
, and the Wien displacement law. For the case of the presence of matter, quantum mechanics provides a good account, as found below in the section headed Einstein coefficients. This was the case considered by Einstein, and is nowadays used for quantum optics. For the case of the absence of matter, quantum field theory is necessary, because non-relativistic quantum mechanics with fixed particle numbers does not provide a sufficient account.
Photons
Quantum theoretical explanation of Planck's law views the radiation as a gas of massless, uncharged, bosonic particles, namely photons, in thermodynamic equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable ...
. Photons are viewed as the carriers of the electromagnetic interaction between electrically charged elementary particles. Photon numbers are not conserved. Photons are created or annihilated in the right numbers and with the right energies to fill the cavity with photons described by the Planck distribution. For a photon gas in thermodynamic equilibrium, the internal energy density is entirely determined by the temperature; moreover, the pressure is entirely determined by the internal energy density. This is unlike the case of thermodynamic equilibrium for material gases, for which the internal energy is determined not only by the temperature, but also, independently, by the respective numbers of the different molecules, and independently again, by the specific characteristics of the different molecules. For different material gases at given temperature, the pressure and internal energy density can vary independently, because different molecules can carry independently different excitation energies.
Planck's law arises as a limit of the Bose–Einstein distribution, the energy distribution describing non-interactive bosons
In particle physics, a boson ( ) is a subatomic particle whose spin quantum number has an integer value (0, 1, 2, ...). Bosons form one of the two fundamental classes of subatomic particle, the other being fermions, which have half odd-integer ...
in thermodynamic equilibrium. In the case of massless bosons such as photons
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that ...
and gluons
A gluon ( ) is a type of massless elementary particle that mediates the strong interaction between quarks, acting as the exchange particle for the interaction. Gluons are massless vector bosons, thereby having a spin of 1. Through the s ...
, the chemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
is zero and the Bose–Einstein distribution reduces to the Planck distribution. There is another fundamental equilibrium energy distribution: the Fermi–Dirac distribution, which describes fermions, such as electrons, in thermal equilibrium. The two distributions differ because multiple bosons can occupy the same quantum state, while multiple fermions cannot. At low densities, the number of available quantum states per particle is large, and this difference becomes irrelevant. In the low density limit, the Bose–Einstein and the Fermi–Dirac distribution each reduce to the Maxwell–Boltzmann distribution
In physics (in particular in statistical mechanics), the Maxwell–Boltzmann distribution, or Maxwell(ian) distribution, is a particular probability distribution named after James Clerk Maxwell and Ludwig Boltzmann.
It was first defined and use ...
.
Kirchhoff's law of thermal radiation
Kirchhoff's law of thermal radiation is a succinct and brief account of a complicated physical situation. The following is an introductory sketch of that situation, and is very far from being a rigorous physical argument. The purpose here is only to summarize the main physical factors in the situation, and the main conclusions.
Spectral dependence of thermal radiation
There is a difference between conductive heat transfer and radiative heat transfer. Radiative heat transfer can be filtered to pass only a definite band of radiative frequencies.
It is generally known that the hotter a body becomes, the more heat it radiates at every frequency.
In a cavity in an opaque body with rigid walls that are not perfectly reflective at any frequency, in thermodynamic equilibrium, there is only one temperature, and it must be shared in common by the radiation of every frequency.
One may imagine two such cavities, each in its own isolated radiative and thermodynamic equilibrium. One may imagine an optical device that allows radiative heat transfer between the two cavities, filtered to pass only a definite band of radiative frequencies. If the values of the spectral radiances of the radiations in the cavities differ in that frequency band, heat may be expected to pass from the hotter to the colder. One might propose to use such a filtered transfer of heat in such a band to drive a heat engine. If the two bodies are at the same temperature, the second law of thermodynamics does not allow the heat engine to work. It may be inferred that for a temperature common to the two bodies, the values of the spectral radiances in the pass-band must also be common. This must hold for every frequency band. This became clear to Balfour Stewart and later to Kirchhoff. Balfour Stewart found experimentally that of all surfaces, one of lamp-black emitted the greatest amount of thermal radiation for every quality of radiation, judged by various filters.
Thinking theoretically, Kirchhoff went a little further and pointed out that this implied that the spectral radiance, as a function of radiative frequency, of any such cavity in thermodynamic equilibrium must be a unique universal function of temperature. He postulated an ideal black body that interfaced with its surroundings in just such a way as to absorb all the radiation that falls on it. By the Helmholtz reciprocity principle, radiation from the interior of such a body would pass unimpeded directly to its surroundings without reflection at the interface. In thermodynamic equilibrium, the thermal radiation emitted from such a body would have that unique universal spectral radiance as a function of temperature. This insight is the root of Kirchhoff's law of thermal radiation.
Relation between absorptivity and emissivity
One may imagine a small homogeneous spherical material body labeled at a temperature , lying in a radiation field within a large cavity with walls of material labeled at a temperature . The body emits its own thermal radiation. At a particular frequency , the radiation emitted from a particular cross-section through the centre of in one sense in a direction normal to that cross-section may be denoted , characteristically for the material of . At that frequency , the radiative power from the walls into that cross-section in the opposite sense in that direction may be denoted , for the wall temperature . For the material of , defining the absorptivity as the fraction of that incident radiation absorbed by , that incident energy is absorbed at a rate .
The rate of accumulation of energy in one sense into the cross-section of the body can then be expressed
Kirchhoff's seminal insight, mentioned just above, was that, at thermodynamic equilibrium at temperature , there exists a unique universal radiative distribution, nowadays denoted , that is independent of the chemical characteristics of the materials and , that leads to a very valuable understanding of the radiative exchange equilibrium of any body at all, as follows.
When there is thermodynamic equilibrium at temperature , the cavity radiation from the walls has that unique universal value, so that . Further, one may define the emissivity of the material of the body just so that at thermodynamic equilibrium at temperature , one has .
When thermal equilibrium prevails at temperature , the rate of accumulation of energy vanishes so that . It follows that in thermodynamic equilibrium, when ,
Kirchhoff pointed out that it follows that in thermodynamic equilibrium, when ,
Introducing the special notation for the absorptivity of material at thermodynamic equilibrium at temperature (justified by a discovery of Einstein, as indicated below), one further has the equality
at thermodynamic equilibrium.
The equality of absorptivity and emissivity here demonstrated is specific for thermodynamic equilibrium at temperature and is in general not to be expected to hold when conditions of thermodynamic equilibrium do not hold. The emissivity and absorptivity are each separately properties of the molecules of the material but they depend differently upon the distributions of states of molecular excitation on the occasion, because of a phenomenon known as "stimulated emission", that was discovered by Einstein. On occasions when the material is in thermodynamic equilibrium or in a state known as local thermodynamic equilibrium, the emissivity and absorptivity become equal. Very strong incident radiation or other factors can disrupt thermodynamic equilibrium or local thermodynamic equilibrium. Local thermodynamic equilibrium in a gas means that molecular collisions far outweigh light emission and absorption in determining the distributions of states of molecular excitation.
Kirchhoff pointed out that he did not know the precise character of , but he thought it important that it should be found out. Four decades after Kirchhoff's insight of the general principles of its existence and character, Planck's contribution was to determine the precise mathematical expression of that equilibrium distribution .
Black body
In physics, one considers an ideal black body, here labeled , defined as one that completely absorbs all of the electromagnetic radiation falling upon it at every frequency (hence the term "black"). According to Kirchhoff's law of thermal radiation, this entails that, for every frequency , at thermodynamic equilibrium at temperature , one has , so that the thermal radiation from a black body is always equal to the full amount specified by Planck's law. No physical body can emit thermal radiation that exceeds that of a black body, since if it were in equilibrium with a radiation field, it would be emitting more energy than was incident upon it.
Though perfectly black materials do not exist, in practice a black surface can be accurately approximated.
Lambert's cosine law
As explained by Planck, a radiating body has an interior consisting of matter, and an interface with its contiguous neighbouring material medium, which is usually the medium from within which the radiation from the surface of the body is observed. The interface is not composed of physical matter but is a theoretical conception, a mathematical two-dimensional surface, a joint property of the two contiguous media, strictly speaking belonging to neither separately. Such an interface can neither absorb nor emit, because it is not composed of physical matter; but it is the site of reflection and transmission of radiation, because it is a surface of discontinuity of optical properties. The reflection and transmission of radiation at the interface obey the Stokes–Helmholtz reciprocity principle.
At any point in the interior of a black body located inside a cavity in thermodynamic equilibrium at temperature the radiation is homogeneous, isotropic and unpolarized. A black body absorbs all and reflects none of the electromagnetic radiation incident upon it. According to the Helmholtz reciprocity principle, radiation from the interior of a black body is not reflected at its surface, but is fully transmitted to its exterior. Because of the isotropy of the radiation in the body's interior, the spectral radiance
In radiometry, spectral radiance or specific intensity is the radiance of a surface per unit frequency or wavelength, depending on whether the Spectral radiometric quantity, spectrum is taken as a function of frequency or of wavelength. The Interna ...
of radiation transmitted from its interior to its exterior through its surface is independent of direction.
This is expressed by saying that radiation from the surface of a black body in thermodynamic equilibrium obeys Lambert's cosine law. This means that the spectral flux from a given infinitesimal element of area of the actual emitting surface of the black body, detected from a given direction that makes an angle with the normal to the actual emitting surface at , into an element of solid angle of detection centred on the direction indicated by , in an element of frequency bandwidth , can be represented as
where denotes the flux, per unit area per unit frequency per unit solid angle, that area would show if it were measured in its normal direction .
The factor is present because the area to which the spectral radiance refers directly is the projection, of the actual emitting surface area, onto a plane perpendicular to the direction indicated by . This is the reason for the name ''cosine law''.
Taking into account the independence of direction of the spectral radiance of radiation from the surface of a black body in thermodynamic equilibrium, one has and so
Thus Lambert's cosine law expresses the independence of direction of the spectral radiance of the surface of a black body in thermodynamic equilibrium.
Stefan–Boltzmann law
The total power emitted per unit area at the surface of a black body () may be found by integrating the black body spectral flux found from Lambert's law over all frequencies, and over the solid angles corresponding to a hemisphere () above the surface.
The infinitesimal solid angle can be expressed in spherical polar coordinates
In mathematics, a spherical coordinate system specifies a given point in three-dimensional space by using a distance and two angles as its three coordinates. These are
* the radial distance along the line connecting the point to a fixed point ...
:
So that:
where is known as the Stefan–Boltzmann constant.
Radiative transfer
The equation of radiative transfer describes the way in which radiation is affected as it travels through a material medium. For the special case in which the material medium is in thermodynamic equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable ...
in the neighborhood of a point in the medium, Planck's law is of special importance.
For simplicity, we can consider the linear steady state, without scattering
In physics, scattering is a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities (including particles and radiat ...
. The equation of radiative transfer states that for a beam of light going through a small distance , energy is conserved: The change in the (spectral) radiance
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiati ...
of that beam () is equal to the amount removed by the material medium plus the amount gained from the material medium. If the radiation field is in equilibrium with the material medium, these two contributions will be equal. The material medium will have a certain emission coefficient and absorption coefficient
The linear attenuation coefficient, attenuation coefficient, or narrow-beam attenuation coefficient characterizes how easily a volume of material can be penetrated by a beam of light, sound, particles, or other energy or matter. A coefficient val ...
.
The absorption coefficient is the fractional change in the intensity of the light beam as it travels the distance , and has units of length−1. It is composed of two parts, the decrease due to absorption and the increase due to stimulated emission
Stimulated emission is the process by which an incoming photon of a specific frequency can interact with an excited atomic electron (or other excited molecular state), causing it to drop to a lower energy level. The liberated energy transfers to ...
. Stimulated emission is emission by the material body which is caused by and is proportional to the incoming radiation. It is included in the absorption term because, like absorption, it is proportional to the intensity of the incoming radiation. Since the amount of absorption will generally vary linearly as the density of the material, we may define a "mass absorption coefficient" which is a property of the material itself. The change in intensity of a light beam due to absorption as it traverses a small distance will then be
Einstein coefficients
The principle of detailed balance
The principle of detailed balance can be used in Kinetics (physics), kinetic systems which are decomposed into elementary processes (collisions, or steps, or elementary reactions). It states that at Thermodynamic equilibrium, equilibrium, each elem ...
states that, at thermodynamic equilibrium, each elementary process is in equilibrium with its reverse process.
In 1916, Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
applied this principle on an atomic level to the case of an atom radiating and absorbing radiation due to transitions between two particular energy levels,
Properties
Peaks
The distributions , , and peak at a photon energy ofLambert W function
In mathematics, the Lambert function, also called the omega function or product logarithm, is a multivalued function, namely the Branch point, branches of the converse relation of the function , where is any complex number and is the expone ...
and is Euler's number
The number is a mathematical constant approximately equal to 2.71828 that is the base of the natural logarithm and exponential function. It is sometimes called Euler's number, after the Swiss mathematician Leonhard Euler, though this can ...
.
However, the distribution peaks at a different energyRiemann zeta function
The Riemann zeta function or Euler–Riemann zeta function, denoted by the Greek letter (zeta), is a mathematical function of a complex variable defined as \zeta(s) = \sum_^\infty \frac = \frac + \frac + \frac + \cdots for and its analytic c ...
.
Approximations
 In the limit of low frequencies (i.e. long wavelengths), Planck's law becomes the
In the limit of low frequencies (i.e. long wavelengths), Planck's law becomes the Rayleigh–Jeans law
In physics, the Rayleigh–Jeans law is an approximation to the spectral radiance of electromagnetic radiation as a function of wavelength from a black body at a given temperature through classical arguments. For wavelength ''λ'', it is
B_\l ...
ultraviolet catastrophe
The ultraviolet catastrophe, also called the Rayleigh–Jeans catastrophe, was the prediction of late 19th century and early 20th century classical physics that an ideal black body at thermal equilibrium would emit an unbounded quantity of en ...
. In the limit of high frequencies (i.e. small wavelengths) Planck's law tends to the Wien approximation
Wien's approximation (also sometimes called Wien's law or the Wien distribution law) is a law of physics used to describe the spectrum of thermal radiation (frequently called the blackbody function). This law was first derived by Wilhelm Wien in ...
:
Percentiles
Wien's displacement law
In physics, Wien's displacement law states that the black-body radiation curve for different temperatures will peak at different wavelengths that are inversely proportional to the temperature. The shift of that peak is a direct consequence of ...
in its stronger form states that the shape of Planck's law is independent of temperature. It is therefore possible to list the percentile
In statistics, a ''k''-th percentile, also known as percentile score or centile, is a score (e.g., a data point) a given percentage ''k'' of all scores in its frequency distribution exists ("exclusive" definition) or a score a given percentage ...
points of the total radiation as well as the peaks for wavelength and frequency, in a form which gives the wavelength when divided by temperature . The second column of the following table lists the corresponding values of , that is, those values of for which the wavelength is micrometers at the radiance percentile point given by the corresponding entry in the first column.
That is, 0.01% of the radiation is at a wavelength below μm, 20% below , etc. The wavelength and frequency peaks are in bold and occur at 25.0% and 64.6% respectively. The 41.8% point is the wavelength-frequency-neutral peak (i.e. the peak in power per unit change in logarithm of wavelength or frequency). These are the points at which the respective Planck-law functions , and , respectively, divided by attain their maxima. The much smaller gap in ratio of wavelengths between 0.1% and 0.01% (1110 is 22% more than 910) than between 99.9% and 99.99% (113374 is 120% more than 51613) reflects the exponential decay of energy at short wavelengths (left end) and polynomial decay at long.
Which peak to use depends on the application. The conventional choice is the wavelength peak at 25.0% given by Wien's displacement law
In physics, Wien's displacement law states that the black-body radiation curve for different temperatures will peak at different wavelengths that are inversely proportional to the temperature. The shift of that peak is a direct consequence of ...
in its weak form. For some purposes the median or 50% point dividing the total radiation into two-halves may be more suitable. The latter is closer to the frequency peak than to the wavelength peak because the radiance drops exponentially at short wavelengths and only polynomially at long. The neutral peak occurs at a shorter wavelength than the median for the same reason.

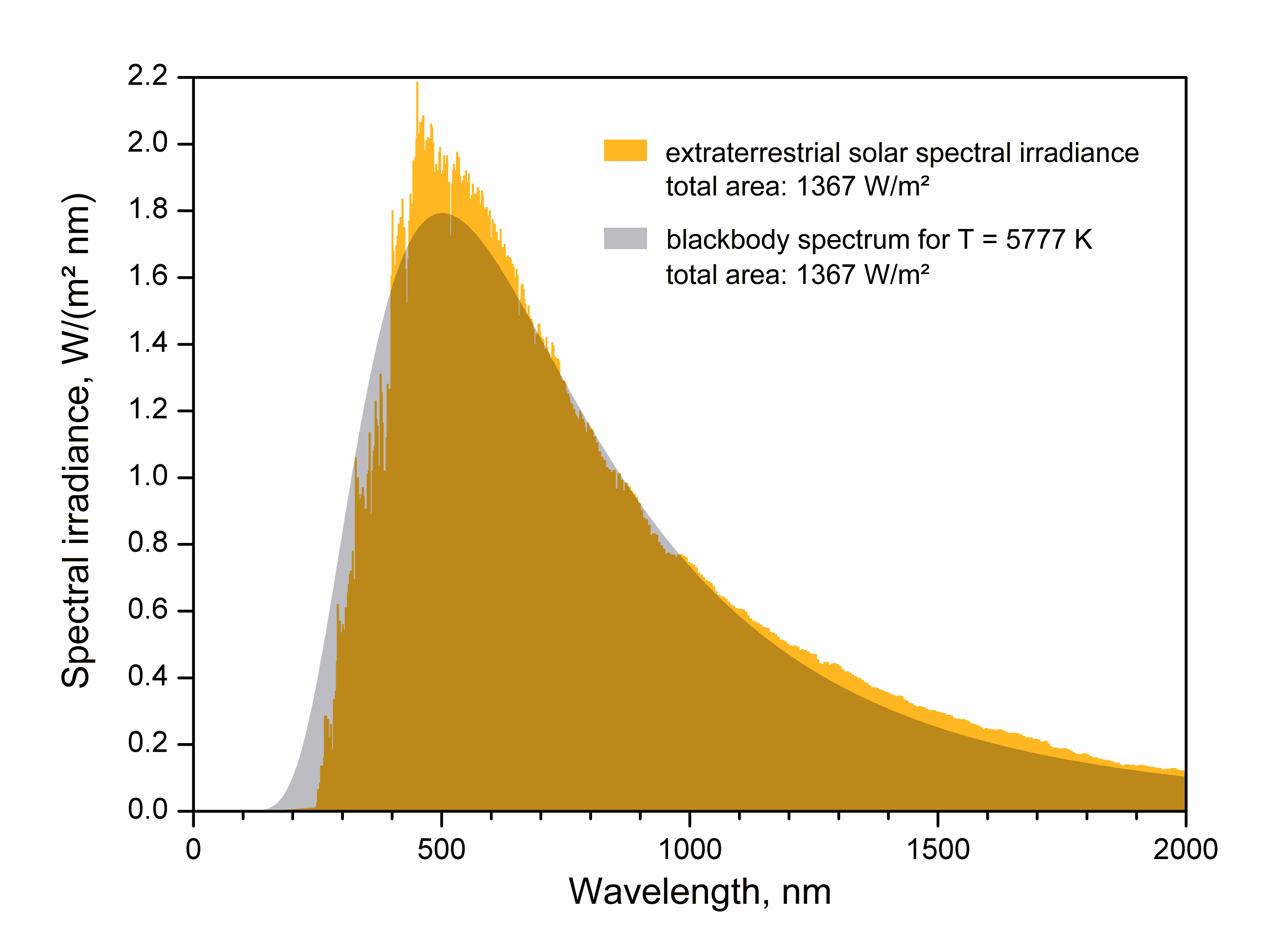
Comparison to solar spectrum
Solar radiation can be compared to black-body radiation at about 5778 K (but see graph). The table on the right shows how the radiation of a black body at this temperature is partitioned, and also how sunlight is partitioned for comparison. Also for comparison a planet modeled as a black body is shown, radiating at a nominal 288 K (15 °C) as a representative value of the Earth's highly variable temperature. Its wavelengths are more than twenty times that of the Sun, tabulated in the third column in micrometers (thousands of nanometers).
That is, only 1% of the Sun's radiation is at wavelengths shorter than 296 nm, and only 1% at longer than 3728 nm. Expressed in micrometers this puts 98% of the Sun's radiation in the range from 0.296 to 3.728 μm. The corresponding 98% of energy radiated from a 288 K planet is from 5.03 to 79.5 μm, well above the range of solar radiation (or below if expressed in terms of frequencies instead of wavelengths ).
A consequence of this more-than-order-of-magnitude difference in wavelength between solar and planetary radiation is that filters designed to pass one and block the other are easy to construct. For example, windows fabricated of ordinary glass or transparent plastic pass at least 80% of the incoming 5778 K solar radiation, which is below 1.2 μm in wavelength, while blocking over 99% of the outgoing 288 K thermal radiation from 5 μm upwards, wavelengths at which most kinds of glass and plastic of construction-grade thickness are effectively opaque.
The Sun's radiation is that arriving at the top of the atmosphere (TOA). As can be read from the table, radiation below 400 nm, or ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
, is about 8%, while that above 700 nm, or infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
, starts at about the 48% point and so accounts for 52% of the total. Hence only 40% of the TOA insolation is visible to the human eye. The atmosphere shifts these percentages substantially in favor of visible light as it absorbs most of the ultraviolet and significant amounts of infrared.
Derivations
Photon gas
Consider a cube of side with conducting walls filled with electromagnetic radiation in thermal equilibrium at temperature . If there is a small hole in one of the walls, the radiation emitted from the hole will be characteristic of a perfect black body. We will first calculate the spectral energy density within the cavity and then determine the spectral radiance of the emitted radiation.
At the walls of the cube, the parallel component of the electric field and the orthogonal component of the magnetic field must vanish. Analogous to the wave function of a particle in a box
In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes the movement of a free particle in a small space surrounded by impenetrable barriers. The model is mainly used a ...
, one finds that the fields are superpositions of periodic functions. The three wavelengths , , and , in the three directions orthogonal to the walls can be:where the are positive integers. For each set of integers there are two linearly independent solutions (known as modes). The two modes for each set of these correspond to the two polarization states of the photon which has a spin of 1. According to quantum theory, the total energy of a mode is given by:
The number can be interpreted as the number of photons in the mode. For the energy of the mode is not zero. This vacuum energy of the electromagnetic field is responsible for the Casimir effect
In quantum field theory, the Casimir effect (or Casimir force) is a physical force (physics), force acting on the macroscopic boundaries of a confined space which arises from the quantum fluctuations of a field (physics), field. The term Casim ...
. In the following we will calculate the internal energy of the box at absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
.
According to statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applicati ...
, the equilibrium probability distribution over the energy levels of a particular mode is given by:where we use the reciprocal temperatureThe denominator , is the partition function of a single mode. It makes properly normalized, and can be evaluated aswith
being the energy of a single photon. The average energy in a mode can be obtained from the partition function:This formula, apart from the first vacuum energy term, is a special case of the general formula for particles obeying Bose–Einstein statistics
In quantum statistics, Bose–Einstein statistics (B–E statistics) describes one of two possible ways in which a collection of non-interacting identical particles may occupy a set of available discrete energy states at thermodynamic equilibri ...
. Since there is no restriction on the total number of photons, the chemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
is zero.
If we measure the energy relative to the ground state, the total energy in the box follows by summing over all allowed single photon states. This can be done exactly in the thermodynamic limit as approaches infinity. In this limit, becomes continuous and we can then integrate over this parameter. To calculate the energy in the box in this way, we need to evaluate how many photon states there are in a given energy range. If we write the total number of single photon states with energies between and as , where is the density of states
In condensed matter physics, the density of states (DOS) of a system describes the number of allowed modes or quantum state, states per unit energy range. The density of states is defined as where N(E)\delta E is the number of states in the syste ...
(which is evaluated below), then the total energy is given by
To calculate the density of states we rewrite equation () as follows:where is the norm of the vector .
For every vector with integer components larger than or equal to zero, there are two photon states. This means that the number of photon states in a certain region of -space is twice the volume of that region. An energy range of corresponds to shell of thickness in -space. Because the components of have to be positive, this shell spans an octant of a sphere. The number of photon states , in an energy range , is thus given by:Inserting this in Eq. () and dividing by volume gives the total energy densitywhere the frequency-dependent spectral energy density is given bySince the radiation is the same in all directions, and propagates at the speed of light, the spectral radiance of radiation exiting the small hole iswhich yields Planck's lawOther forms of the law can be obtained by change of variables in the total energy integral. The above derivation is based on .
Dipole approximation and Einstein Coefficients
For the non-degenerate case, A and B coefficients can be calculated using dipole approximation in time dependent perturbation theory in quantum mechanics. Calculation of A also requires second quantization since semi-classical theory cannot explain spontaneous emission which does not go to zero as the perturbing field goes to zero. Hence, the calculated transition rates are (in SI units):
History
Balfour Stewart
In 1858, Balfour Stewart described his experiments on the thermal radiative emissive and absorptive powers of polished plates of various substances, compared with the powers of lamp-black surfaces, at the same temperature.Pierre Prevost
Pierre is a masculine given name. It is a French form of the name Peter. Pierre originally meant "rock" or "stone" in French (derived from the Greek word πέτρος (''petros'') meaning "stone, rock", via Latin "petra"). It is a translatio ...
and of John Leslie. He wrote "Lamp-black, which absorbs all the rays that fall upon it, and therefore possesses the greatest possible absorbing power, will possess also the greatest possible radiating power."
Stewart measured radiated power with a thermo-pile and sensitive galvanometer read with a microscope. He was concerned with selective thermal radiation, which he investigated with plates of substances that radiated and absorbed selectively for different qualities of radiation rather than maximally for all qualities of radiation. He discussed the experiments in terms of rays which could be reflected and refracted, and which obeyed the Helmholtz reciprocity principle (though he did not use an eponym for it). He did not in this paper mention that the qualities of the rays might be described by their wavelengths, nor did he use spectrally resolving apparatus such as prisms or diffraction gratings. His work was quantitative within these constraints. He made his measurements in a room temperature environment, and quickly so as to catch his bodies in a condition near the thermal equilibrium in which they had been prepared by heating to equilibrium with boiling water. His measurements confirmed that substances that emit and absorb selectively respect the principle of selective equality of emission and absorption at thermal equilibrium.
Stewart offered a theoretical proof that this should be the case separately for every selected quality of thermal radiation, but his mathematics was not rigorously valid. According to historian D. M. Siegel: "He was not a practitioner of the more sophisticated techniques of nineteenth-century mathematical physics; he did not even make use of the functional notation in dealing with spectral distributions."
Gustav Kirchhoff
In 1859, not knowing of Stewart's work, Gustav Robert Kirchhoff
Gustav Robert Kirchhoff (; 12 March 1824 – 17 October 1887) was a German chemist, mathematician, physicist, and spectroscopist who contributed to the fundamental understanding of electrical circuits, spectroscopy and the emission of black-body ...
reported the coincidence of the wavelengths of spectrally resolved lines of absorption and of emission of visible light. Importantly for thermal physics, he also observed that bright lines or dark lines were apparent depending on the temperature difference between emitter and absorber.
Kirchhoff then went on to consider bodies that emit and absorb heat radiation, in an opaque enclosure or cavity, in equilibrium at temperature .
Here is used a notation different from Kirchhoff's. Here, the emitting power denotes a dimensioned quantity, the total radiation emitted by a body labeled by index at temperature . The total absorption ratio of that body is dimensionless, the ratio of absorbed to incident radiation in the cavity at temperature . (In contrast with Balfour Stewart's, Kirchhoff's definition of his absorption ratio did not refer in particular to a lamp-black surface as the source of the incident radiation.) Thus the ratio of emitting power to absorption ratio is a dimensioned quantity, with the dimensions of emitting power, because is dimensionless. Also here the wavelength-specific emitting power of the body at temperature is denoted by and the wavelength-specific absorption ratio by . Again, the ratio of emitting power to absorption ratio is a dimensioned quantity, with the dimensions of emitting power.
In a second report made in 1859, Kirchhoff announced a new general principle or law for which he offered a theoretical and mathematical proof, though he did not offer quantitative measurements of radiation powers. His theoretical proof was and still is considered by some writers to be invalid.
Empirical and theoretical ingredients for the scientific induction of Planck's law
In 1860, Kirchhoff predicted experimental difficulties for the empirical determination of the function that described the dependence of the black-body spectrum as a function only of temperature and wavelength. And so it turned out. It took some forty years of development of improved methods of measurement of electromagnetic radiation to get a reliable result.Ferdinand Kurlbaum
Ferdinand Kurlbaum (4 October 1857 in Burg bei Magdeburg – 29 July 1927 in Berlin) was a German people, German physicist.
Life and work
As the son of a magistrate, he had to follow his frequently transferred father. Problems at school wer ...
published an account of their cavity radiation source. Their design has been used largely unchanged for radiation measurements to the present day. It was a platinum box, divided by diaphragms, with its interior blackened with iron oxide. It was an important ingredient for the progressively improved measurements that led to the discovery of Planck's law. A version described in 1901 had its interior blackened with a mixture of chromium, nickel, and cobalt oxides.
The importance of the Lummer and Kurlbaum cavity radiation source was that it was an experimentally accessible source of black-body radiation, as distinct from radiation from a simply exposed incandescent solid body, which had been the nearest available experimental approximation to black-body radiation over a suitable range of temperatures. The simply exposed incandescent solid bodies, that had been used before, emitted radiation with departures from the black-body spectrum that made it impossible to find the true black-body spectrum from experiments.
Planck's views before the empirical facts led him to find his eventual law
Planck first turned his attention to the problem of black-body radiation in 1897.[, p. 460.]
Theoretical and empirical progress enabled Lummer and Pringsheim to write in 1899 that available experimental evidence was approximately consistent with the specific intensity law where and denote empirically measurable constants, and where and denote wavelength and temperature respectively. For theoretical reasons, Planck at that time accepted this formulation, which has an effective cut-off of short wavelengths.Ferdinand Kurlbaum
Ferdinand Kurlbaum (4 October 1857 in Burg bei Magdeburg – 29 July 1927 in Berlin) was a German people, German physicist.
Life and work
As the son of a magistrate, he had to follow his frequently transferred father. Problems at school wer ...
, Ernst Pringsheim Sr., and Heinrich Rubens did experiments that appeared to support Wien's law especially at higher frequency, i.e. short wavelengths, which Planck so wholly endorsed at the German Physical Society that it began to be called the Wien-Planck Law. However, by September 1900, the experimentalists had proven beyond a doubt that the Wien-Planck law failed at the longer wavelengths. They would present their data on October 19. Planck was informed by his friend Rubens and quickly created a formula within a few days. In June of that same year, Lord Rayleigh had created a formula that would work for lower frequency based on the widely accepted theory of equipartition. So Planck submitted a formula combining both Rayleigh's Law (or a similar equipartition theory) and Wien's law which would be weighted to one or the other law depending on wavelength to match the experimental data. However, although this equation worked, Planck himself said unless he could explain the formula derived from a "lucky intuition" into one of "true meaning" in physics, it did not have true significance. Planck explained that thereafter followed the hardest work of his life. Planck did not believe in atoms, nor did he think the second law of thermodynamics should be statistical because probability does not provide an absolute answer, and Boltzmann's entropy law rested on the hypothesis of atoms and was statistical. But Planck was unable to find a way to reconcile his blackbody equation with continuous laws such as Maxwell's wave equations. So in what Planck called "an act of desperation", he turned to Boltzmann's atomic law of entropy as it was the only one that made his equation work. Therefore, he used the Boltzmann constant ''k'' and his new auxiliary constant ''h'' to explain the blackbody radiation law which later became widely known through his published paper.
Finding the empirical law
Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
produced his law on 19 October 1900Wien approximation
Wien's approximation (also sometimes called Wien's law or the Wien distribution law) is a law of physics used to describe the spectrum of thermal radiation (frequently called the blackbody function). This law was first derived by Wilhelm Wien in ...
, published in 1896 by Wilhelm Wien, which fit the experimental data at short wavelengths (high frequencies) but deviated from it at long wavelengths (low frequencies).heuristic
A heuristic or heuristic technique (''problem solving'', '' mental shortcut'', ''rule of thumb'') is any approach to problem solving that employs a pragmatic method that is not fully optimized, perfected, or rationalized, but is nevertheless ...
theoretical considerations, Rayleigh had suggested a formula
Trying to find a physical explanation of the law
Once Planck had discovered the empirically fitting function, he constructed a physical derivation of this law. His thinking revolved around entropy rather than being directly about temperature. Planck considered a cavity with perfectly reflective walls; inside the cavity, there are finitely many distinct but identically constituted resonant oscillatory bodies of definite magnitude, with several such oscillators at each of finitely many characteristic frequencies. These hypothetical oscillators were for Planck purely imaginary theoretical investigative probes, and he said of them that such oscillators do not need to "really exist somewhere in nature, provided their existence and their properties are consistent with the laws of thermodynamics and electrodynamics.". Planck did not attribute any definite physical significance to his hypothesis of resonant oscillators but rather proposed it as a mathematical device that enabled him to derive a single expression for the black body spectrum that matched the empirical data at all wavelengths. He tentatively mentioned the possible connection of such oscillators with atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s. In a sense, the oscillators corresponded to Planck's speck of carbon; the size of the speck could be small regardless of the size of the cavity, provided the speck effectively transduced energy between radiative wavelength modes.Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
.
Planck explained furtherquantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines Field theory (physics), field theory and the principle of relativity with ideas behind quantum mechanics. QFT is used in particle physics to construct phy ...
.
In 1906, Planck acknowledged that his imaginary resonators, having linear dynamics, did not provide a physical explanation for energy transduction between frequencies. Present-day physics explains the transduction between frequencies in the presence of atoms by their quantum excitability, following Einstein. Planck believed that in a cavity with perfectly reflecting walls and with no matter present, the electromagnetic field cannot exchange energy between frequency components.linearity
In mathematics, the term ''linear'' is used in two distinct senses for two different properties:
* linearity of a '' function'' (or '' mapping'');
* linearity of a '' polynomial''.
An example of a linear function is the function defined by f(x) ...
of Maxwell's equations
Maxwell's equations, or Maxwell–Heaviside equations, are a set of coupled partial differential equations that, together with the Lorentz force law, form the foundation of classical electromagnetism, classical optics, Electrical network, electr ...
.[, Chapter 13]Gustav Kirchhoff
Gustav Robert Kirchhoff (; 12 March 1824 – 17 October 1887) was a German chemist, mathematician, physicist, and spectroscopist who contributed to the fundamental understanding of electrical circuits, spectroscopy and the emission of black-body ...
that his law of thermal radiation was of the highest importance. In his mature presentation of his own law, Planck offered a thorough and detailed theoretical proof for Kirchhoff's law, theoretical proof of which until then had been sometimes debated, partly because it was said to rely on unphysical theoretical objects, such as Kirchhoff's perfectly absorbing infinitely thin black surface.
Subsequent events
It was not until five years after Planck made his heuristic assumption of abstract elements of energy or of action that Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
conceived of really existing quanta of light in 1905photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
, and of the ionization of gases by ultraviolet light. In 1905, "Einstein believed that Planck's theory could not be made to agree with the idea of light quanta, a mistake he corrected in 1906." Contrary to Planck's beliefs of the time, Einstein proposed a model and formula whereby light was emitted, absorbed, and propagated in free space in energy quanta localized in points of space.Thomas Kuhn
Thomas Samuel Kuhn (; July 18, 1922 – June 17, 1996) was an American History and philosophy of science, historian and philosopher of science whose 1962 book ''The Structure of Scientific Revolutions'' was influential in both academic and ...
, it was not till 1908 that Planck more or less accepted part of Einstein's arguments for physical as distinct from abstract mathematical discreteness in thermal radiation physics. Still in 1908, considering Einstein's proposal of quantal propagation, Planck opined that such a revolutionary step was perhaps unnecessary. Until then, Planck had been consistent in thinking that discreteness of action quanta was to be found neither in his resonant oscillators nor in the propagation of thermal radiation. Kuhn wrote that, in Planck's earlier papers and in his 1906 monograph,ultraviolet catastrophe
The ultraviolet catastrophe, also called the Rayleigh–Jeans catastrophe, was the prediction of late 19th century and early 20th century classical physics that an ideal black body at thermal equilibrium would emit an unbounded quantity of en ...
" was given by Paul Ehrenfest
Paul Ehrenfest (; 18 January 1880 – 25 September 1933) was an Austrian Theoretical physics, theoretical physicist who made major contributions to statistical mechanics and its relation to quantum physics, quantum mechanics, including the theory ...
in 1911 to the paradoxical result that the total energy in the cavity tends to infinity when the equipartition theorem
In classical physics, classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energy, energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, ...
of classical statistical mechanics is (mistakenly) applied to black-body radiation. But this had not been part of Planck's thinking, because he had not tried to apply the doctrine of equipartition: when he made his discovery in 1900, he had not noticed any sort of "catastrophe".James Jeans
Sir James Hopwood Jeans (11 September 1877 – 16 September 1946) was an English physicist, mathematician and an astronomer. He served as a secretary of the Royal Society from 1919 to 1929, and was the president of the Royal Astronomical Soci ...
; and later, in 1905, by Einstein when he wanted to support the idea that light propagates as discrete packets, later called 'photons', and by RayleighSatyendra Nath Bose
Satyendra Nath Bose (; 1 January 1894 – 4 February 1974) was an Indian theoretical physicist and mathematician. He is best known for his work on quantum mechanics in the early 1920s, in developing the foundation for Bose–Einstein statist ...
developed the theory of the statistical mechanics of photons, which allowed a theoretical derivation of Planck's law. The actual word 'photon' was invented still later, by G.N. Lewis in 1926, who mistakenly believed that photons were conserved, contrary to Bose–Einstein statistics; nevertheless the word 'photon' was adopted to express the Einstein postulate of the packet nature of light propagation. In an electromagnetic field isolated in a vacuum in a vessel with perfectly reflective walls, such as was considered by Planck, indeed the photons would be conserved according to Einstein's 1905 model, but Lewis was referring to a field of photons considered as a system closed with respect to ponderable matter but open to exchange of electromagnetic energy with a surrounding system of ponderable matter, and he mistakenly imagined that still the photons were conserved, being stored inside atoms.
Ultimately, Planck's law of black-body radiation contributed to Einstein's concept of quanta of light carrying linear momentum,quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
.
The above-mentioned linearity of Planck's mechanical assumptions, not allowing for energetic interactions between frequency components, was superseded in 1925 by Heisenberg's original quantum mechanics. In his paper submitted on 29 July 1925, Heisenberg's theory accounted for Bohr's above-mentioned formula of 1913. It admitted non-linear oscillators as models of atomic quantum states, allowing energetic interaction between their own multiple internal discrete Fourier frequency components, on the occasions of emission or absorption of quanta of radiation. The frequency of a quantum of radiation was that of a definite coupling between internal atomic meta-stable oscillatory quantum states. At that time, Heisenberg knew nothing of matrix algebra, but Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a ...
read the manuscript of Heisenberg's paper and recognized the matrix character of Heisenberg's theory. Then Born and Jordan
Jordan, officially the Hashemite Kingdom of Jordan, is a country in the Southern Levant region of West Asia. Jordan is bordered by Syria to the north, Iraq to the east, Saudi Arabia to the south, and Israel and the occupied Palestinian ter ...
published an explicitly matrix theory of quantum mechanics, based on, but in form distinctly different from, Heisenberg's original quantum mechanics; it is the Born and Jordan matrix theory that is today called matrix mechanics. Heisenberg's explanation of the Planck oscillators, as non-linear effects apparent as Fourier modes of transient processes of emission or absorption of radiation, showed why Planck's oscillators, viewed as enduring physical objects such as might be envisaged by classical physics, did not give an adequate explanation of the phenomena.
Nowadays, as a statement of the energy of a light quantum, often one finds the formula , where , and denotes angular frequency,Physics World
''Physics World'' is the membership magazine of the Institute of Physics, one of the largest physical societies in the world. It is an international monthly magazine covering all areas of physics, pure and applied, and is aimed at physicists in ...
'' gives an account of this history.
See also
* Emissivity
The emissivity of the surface of a material is its effectiveness in emitting energy as thermal radiation. Thermal radiation is electromagnetic radiation that most commonly includes both visible radiation (light) and infrared radiation, which is n ...
* Radiance
In radiometry, radiance is the radiant flux emitted, reflected, transmitted or received by a given surface, per unit solid angle per unit projected area. Radiance is used to characterize diffuse emission and reflection of electromagnetic radiati ...
* Sakuma–Hattori equation
In physics, the Sakuma–Hattori equation is a mathematical model for predicting the amount of thermal radiation, radiometric flux or radiometric power emitted from a perfect blackbody or received by a thermal radiation detector.
History
The ...
References
Bibliography
*
*
*
*
*
*
*
** Translated in part as "On quantum mechanics" in
*
*
*
*
*
*
*
*
*
*
*
** Translated in
* and a nearly identical version
** Translated in
** See als
*
*
*
*
*
*
*
*
*
** Translated as "Quantum-theoretical Re-interpretation of kinematic and mechanical relations" in
*
*
** Translated from ''Frühgeschichte der Quantentheorie (1899–1913)'', Physik Verlag, Mosbach/Baden, 1969.
*
*
*
*
*
*
*
*
*
*
*
*
*
*
** Translated by Guthrie, F. as
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
** Translated in
*
** Translated in
*
*
*
** Translated in
*
*
*
*
*
*
*
*
*
** Translated in
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
External links
Summary of Radiation
– interactive simulation to play with Planck's law
{{DEFAULTSORT:Planck's Law
Statistical mechanics
Foundational quantum physics
Max Planck
Old quantum theory
1900 in science
1900 in Germany
 A black-body is an idealised object which absorbs and emits all radiation frequencies. Near
A black-body is an idealised object which absorbs and emits all radiation frequencies. Near 