Pictet–Spengler Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Pictet–Spengler reaction is a
The reaction of
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
in which a β-arylethylamine undergoes condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
with an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet
Amé Jules Pictet (12 July 1857 – 11 March 1937) was a Swiss chemist. He discovered the Pictet–Spengler reaction, and the related Pictet–Hubert reaction and Pictet–Gams reaction. He is credited with publishing the first synthesis of n ...
and Theodor Spengler (22 February 1886 – 18 August 1965). Traditionally, an acidic catalyst in protic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labi ...
was employed with heating; however, the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By Brønsted–Lowry acid–base theory, the acid is the proton (hydrogen ion, H+) donor and the base is the proton acceptor. Typical reactions catalyz ...
. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia () ...
, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
of the iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries: the central C=N unit is nearly coplanar with a ...
ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.
The Pictet–Spengler reaction is widespread in both industry and biosynthesis. It has remained an important reaction in the fields of alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
and organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
since its inception, where it has been employed in the development of many beta-carbolines. Natural Pictet–Spengler reaction typically employ an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
, such as strictosidine synthase
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:
:3-α(''S'')-strictosidine + H2O = tryptamine ...
. Pictet–Spengler products can be isolated from many products initially derived from nature, including foodstuffs such as soy sauce
Soy sauce (sometimes called soya sauce in British English) is a liquid condiment of China, Chinese origin, traditionally made from a fermentation (food), fermented paste of soybeans, roasted cereal, grain, brine, and ''Aspergillus oryzae'' or ''A ...
and ketchup
Ketchup or catsup is a table condiment with a sweet and sour flavor. "Ketchup" now typically refers to tomato ketchup, although early recipes for different varieties contained mushrooms, oysters, mussels, egg whites, grapes, or walnuts, amon ...
. In such cases it is common to find the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
and various aldose
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ket ...
s used as the biological feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials/Intermediate goods that are feedstock for future finishe ...
.
Nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
rings such as indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole ...
or pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrol ...
give products in high yields and mild conditions, while less nucleophilic aromatic rings such as a phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
group give poorer yields or require higher temperatures and strong acid. The original Pictet–Spengler reaction was the reaction of phenethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace ami ...
and dimethoxymethane
Dimethoxymethane, also called methylal, is a colorless flammable liquid with a low boiling point, low viscosity and excellent dissolving power. It has a chloroform-like odor and a pungent taste. It is the dimethyl acetal
In organic chemis ...
, catalysed by hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
forming a tetrahydroisoquinoline
Tetrahydroisoquinoline (TIQ or THIQ), also known as AMPH-CR, is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is mis ...
.
The Pictet–Spengler reaction has been applied to solid-phase combinatorial chemistry
Combinatorial chemistry comprises chemical synthesis, chemical synthetic methods that make it possible to prepare a large number (tens to thousands or even millions) of compounds in a single process. These compound library, compound libraries can b ...
with great success.
An analogous reaction with an aryl-β-ethanol is called oxa-Pictet–Spengler reaction.
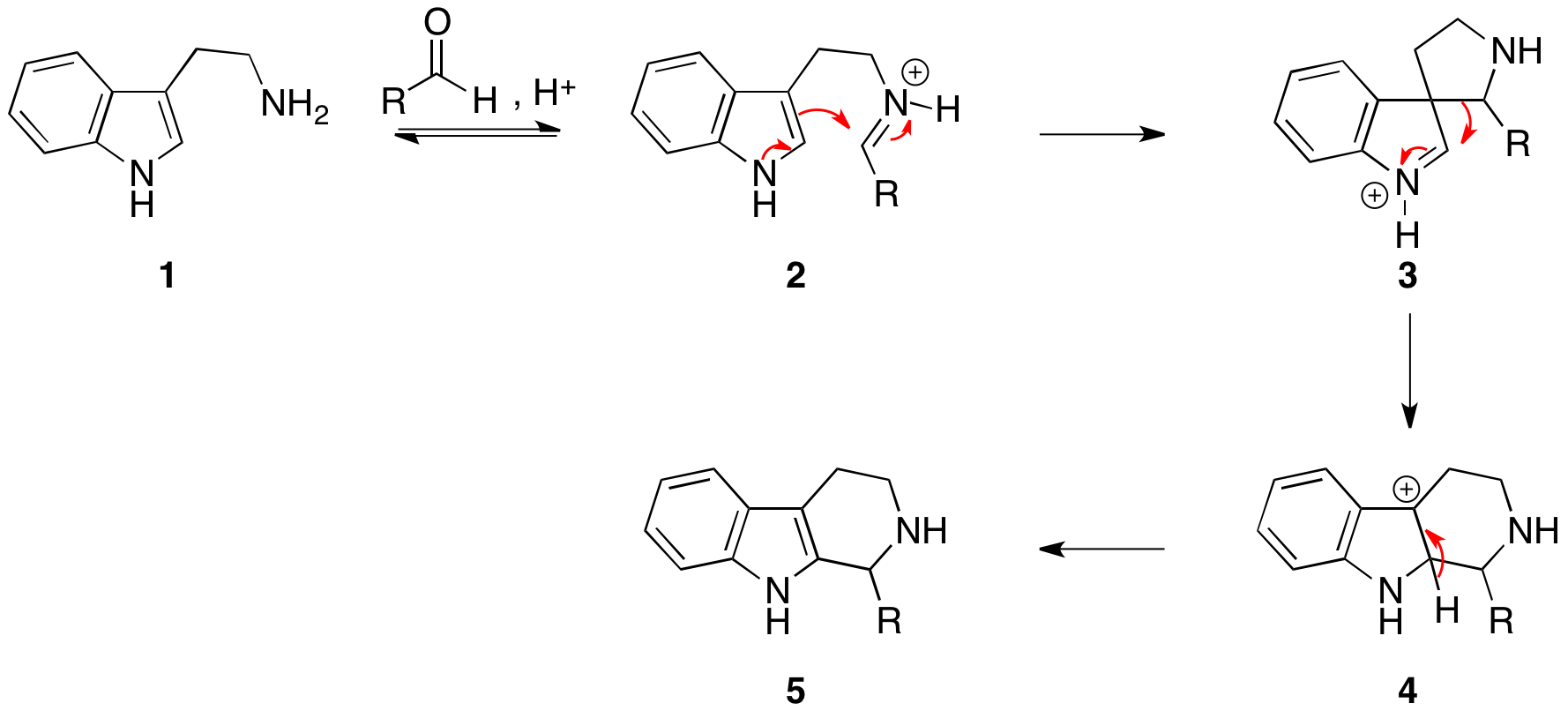
Reaction mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
occurs by initial formation of an iminium ion (2) followed by electrophilic addition
In organic chemistry, an electrophilic addition (AE) reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic ...
at the 3-position, in accordance with the expected nucleophilicity of indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole ...
s, to give the spirocycle 3. After migration of the best migrating group, deprotonation gives the product (5).

Variations
Pictet–Spengler tetrahydroisoquinoline synthesis
Replacing an indole with a 3,4-dimethoxyphenyl group give the reaction named the Pictet–Spengler tetrahydroisoquinoline synthesis. Reaction conditions are generally harsher than the indole variant, and require refluxing conditions with strong acids likehydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
, trifluoroacetic acid
Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not ...
or superacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid i ...
s.
''N''-acyliminium ion Pictet–Spengler reaction
Instead of catalyzing the Pictet–Spengler cyclization with strong acid, one can acylate the iminium ion forming the intermediate ''N''-acyliminium ion. The ''N''-acyliminium ion is a very powerfulelectrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
and most aromatic ring systems will cyclize under mild conditions with good yields.
Tadalafil
Tadalafil, sold under the brand name Cialis among others, is a medication used to treat erectile dysfunction, benign prostatic hyperplasia, and pulmonary arterial hypertension. It is taken by mouth. Onset is typically within half an hour and ...
is synthesized via the ''N''-acyliminium Pictet–Spengler reaction. This reaction can also be catalyzed by AuCl3 and AgOTf.
Asymmetric Pictet–Spengler reaction
When the Pictet–Spengler reaction is performed with an aldehyde other thanformaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
, a new chiral center is created. Several substrate- or auxiliary-controlled diastereoselective
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
Pictet–Spengler reactions have been developed. Additionally, List ''et al.'' have published a chiral Brønsted–Lowry acid that catalyzes asymmetric Pictet–Spengler reactions.
Tryptophans: diastereocontrolled reactionThe reaction of
enantiopure
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities which are mirror images of each other and non-superpos ...
tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
or its short-chain alkylester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s leads to 1,2,3,4-tetrahydro- ''β''-carbolines in which a new chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
center at C-1 adopts either a '' cis'' or ''trans'' configuration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice board ...
towards the C-3 carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
group. The ''cis'' conduction is kinetically controlled, i.e. it is performed at lower temperatures. At higher temperatures the reaction becomes reversible and usually favours racemisation. 1,3-''trans'' dominated products can be obtained with ''Nb''-benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group ().
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substituent ...
ated tryptophans, which are accessible by reductive amination
Reductive amination (also known as reductive alkylation) is a form of amination that converts a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is a common method to make amine ...
. The benzyl group can be removed hydrogenolytically afterwards. As a rough rule, 13C NMR signals for C1 and C3 are downfield shifted in ''cis'' products relative to ''trans'' products (see steric compression effect).
See also
*Bischler–Napieralski reaction
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and , in affi ...
* Pomeranz–Fritsch reaction
The Pomeranz–Fritsch reaction, also named Pomeranz–Fritsch cyclization, is a named reaction in organic chemistry. It is named after Paul Fritsch (chemist), Paul Fritsch (1859–1913) and Cäsar Pomeranz (1860–1926). In general it is a synth ...
References
{{DEFAULTSORT:Pictet-Spengler Reaction Condensation reactions Heterocycle forming reactions Name reactions