Octet Rule on:
[Wikipedia]
[Google]
[Amazon]
 The octet rule is a
The octet rule is a
 The octet rule is simplest in the case of
The octet rule is simplest in the case of
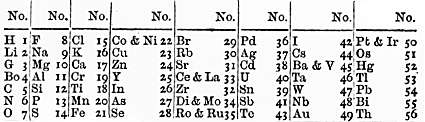 In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893, Alfred Werner showed that the number of atoms or groups associated with a central atom (the "
In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893, Alfred Werner showed that the number of atoms or groups associated with a central atom (the "
 The octet rule is a
The octet rule is a chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
rule of thumb
In English language, English, the phrase ''rule of thumb'' refers to an approximate method for doing something, based on practical experience rather than theory. This usage of the phrase can be traced back to the 17th century and has been associat ...
that reflects the theory that main-group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
s tend to bond in such a way that each atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
has eight electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
in its valence shell, giving it the same electronic configuration as a noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
. The rule is especially applicable to carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, and the halogens
The halogens () are a group (periodic table), group in the periodic table consisting of six chemically related chemical element, elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and ten ...
; although more generally the rule is applicable for the s-block and p-block of the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. Other rules exist for other elements, such as the duplet rule for hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
and helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, and the 18-electron rule for transition metals.
The valence electrons in molecules like carbon dioxide (CO₂) can be visualized using a Lewis electron dot diagram. In covalent bonds, electrons shared between two atoms are counted toward the octet of both atoms. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon octet and the oxygen octet, so that both atoms are considered to obey the octet rule.
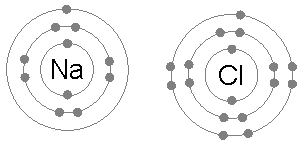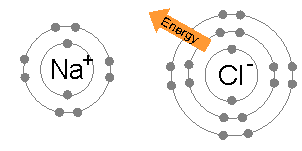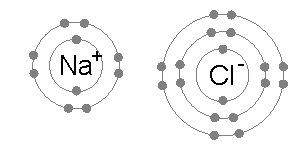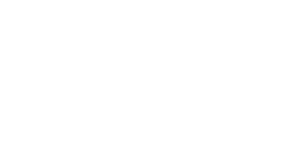
Example: sodium chloride (NaCl)
 The octet rule is simplest in the case of
The octet rule is simplest in the case of ionic bonding
Ionic bonding is a type of chemical bonding that involves the Coulomb's law, electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in io ...
between two atoms, one a metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
of low electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
and the other a nonmetal of high electronegativity. For example, sodium metal and chlorine gas combine to form sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
, a crystal lattice
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystal, crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that ...
composed of alternating sodium and chlorine nuclei. Electron density inside this lattice forms clumps at the atomic scale, as follows.
An isolated chlorine atom (Cl) has two and eight electrons in its first and second electron shells, located near the nucleus. However, it has only seven electrons in the third and outermost electron shell. One additional electron would completely fill the outer electron shell with eight electrons, a situation the octet rule commends. Indeed, adding an electron to the produce the chloride ion (Cl−) releases 3.62 eV of energy. Conversely, another surplus electron cannot fit in the same shell, instead beginning the fourth electron shell around the nucleus. Thus the octet rule proscribes formation of a hypothetical Cl2− ion, and indeed the latter has only been observed as a plasma under extreme conditions.
A sodium atom (Na) has a single electron in its outermost electron shell, the first and second shells again being full with two and eight electrons respectively. The octet rule favors removal of this outermost electron to form the Na+ ion, which has the exact same electron configuration as Cl−. Indeed, sodium is observed to transfer one electron to chlorine during the formation of sodium chloride, such that the resulting lattice is best considered as a periodic array of Na+ and Cl− ions.
To remove the outermost Na electron and return to an "octet-approved" state requires a small amount of energy: 5.14 eV. This energy is provided from the 3.62 eV released during chloride formation, and the electrostatic attraction between positively-charged Na+ and negatively-charged Cl− ions, which releases a 8.12 eV lattice energy. By contrast, any further electrons removed from Na would reside in the deeper second electron shell, and produce an octet-violating Na2+ ion. Consequently, the second ionization energy required for the next removal is much larger — 47.28 eV — and the corresponding ion is only observed under extreme conditions.
History
 In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893, Alfred Werner showed that the number of atoms or groups associated with a central atom (the "
In 1864, the English chemist John Newlands classified the sixty-two known elements into eight groups, based on their physical properties.
In the late 19th century, it was known that coordination compounds (formerly called "molecular compounds") were formed by the combination of atoms or molecules in such a manner that the valencies of the atoms involved apparently became satisfied. In 1893, Alfred Werner showed that the number of atoms or groups associated with a central atom (the "coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
") is often 4 or 6; other coordination numbers up to a maximum of 8 were known, but less frequent. In 1904, Richard Abegg was one of the first to extend the concept of coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
to a concept of valence in which he distinguished atoms as electron donors or acceptors, leading to positive and negative valence states that greatly resemble the modern concept of oxidation states. Abegg noted that the difference between the maximum positive and negative valences of an element under his model is frequently eight. In 1916, Gilbert N. Lewis referred to this insight as Abegg's rule and used it to help formulate his cubical atom model and the "rule of eight", which began to distinguish between valence and valence electrons. In 1919, Irving Langmuir refined these concepts further and renamed them the "cubical octet atom" and "octet theory". The "octet theory" evolved into what is now known as the "octet rule".
Walther Kossel and Gilbert N. Lewis saw that noble gases did not have the tendency of taking part in chemical reactions under ordinary conditions. On the basis of this observation, they concluded that atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s of noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es are stable and on the basis of this conclusion they proposed a theory of valency known as "electronic theory of valency" in 1916:
Explanation in quantum theory
The quantum theory of the atom explains the eight electrons as a closed shell with an s2p6 electron configuration. A closed-shell configuration is one in which low-lying energy levels are full and higher energy levels are empty. For example, theneon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
atom ground state has a full shell (2s22p6) and an empty shell. According to the octet rule, the atoms immediately before and after neon in the periodic table (i.e. C, N, O, F, Na, Mg and Al), tend to attain a similar configuration by gaining, losing, or sharing electrons.
The argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
atom has an analogous 3s23p6 configuration. There is also an empty 3d level, but it is at considerably higher energy than 3s and 3p (unlike in the hydrogen atom), so that 3s23p6 is still considered a closed shell for chemical purposes. The atoms immediately before and after argon tend to attain this configuration in compounds. There are, however, some hypervalent molecules in which the 3d level may play a part in the bonding, although this is controversial (see below).
For helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
there is no 1p level according to the quantum theory, so that 1s2 is a closed shell with no p electrons. The atoms before and after helium (H and Li) follow a duet rule and tend to have the same 1s2 configuration as helium.
Exceptions
Many reactive intermediates do not obey the octet rule. Most are unstable, although some can be isolated. Typically, octet rule violations occur in either low-dimensional coordination geometries or in radical species. Although hypervalent molecules are commonly taught to violate the octet rule, ''ab initio'' calculations show that almost all known examples obey the octet rule. The compounds form many fractional bonds throughresonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
(see below).
Low-dimensional geometries
In the trigonal planar coordination geometry, one ''p'' orbital points out of the bonding plane, and can only overlap with nearby atomic orbitals in a π bond. If that ''p'' orbital would be empty in an isolated atom, it may be filled through an intramolecular dative bond, as with aminoboranes. However, in some cases (e.g. boron trichloride and various boranes, triphenylmethanium), no nearby filled orbital can profitably overlap with the empty ''p'' orbital. In such cases, the orbital remains empty, and the compound obeys a "sextet rule". Likewise, linear compounds, such as dimethylzinc, have two ''p'' orbitals perpendicular to the bonding axis, and may obey a "quartet rule". In either case, the empty unshielded orbitals tend to attract adducts.Radicals
Radicals satisfy the octet rule in one spin orientation, with four spin-up electrons in the valence shell, and almost satisfy it in the opposite spin orientation. Thus, for example, the methyl radical (CH3), which has an unpaired electron in a non-bonding orbital on the carbon atom and no electron of opposite spin in the same orbital. Another example is the radical chlorine monoxide (ClO•) which is involved in ozone depletion. Stable radicals tend to adopt states in which the unpaired electron can delocalize through resonance. In such cases, the octet rule can be restored through the formalism of a 1- or 3-electron bond. Species such as carbenes can be interpreted two different ways, depending on their spin state. Triplet carbenes are best thought of as two radicals localized on the same atom, and obey the octet rule in those radicals' shared spin-up orientation. Singlet carbenes tend to adopt a planar configuration, and are best thought of as obeying the planar sextet rule.Hypervalent molecules
Main-group elements in the third and later rows of the periodic table can form hypercoordinate or hypervalent molecules in which the central main-group atom is bonded to more than four other atoms, such as phosphorus pentafluoride, PF5, and sulfur hexafluoride, SF6. For example, in PF5, if it is supposed that there are five truecovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s in which five distinct electron pairs are shared, then the phosphorus would be surrounded by 10 valence electrons in violation of the octet rule. In the early days of quantum mechanics, Pauling proposed that third-row atoms can form five bonds by using one s, three p and one d orbitals, or six bonds by using one s, three p and two d orbitals. To form five bonds, the one s, three p and one d orbitals combine to form five sp3d hybrid orbitals which each share an electron pair with a halogen atom, for a total of 10 shared electrons, two more than the octet rule predicts. Similarly to form six bonds, the six sp3d2 hybrid orbitals form six bonds with 12 shared electrons. In this model the availability of empty d orbitals is used to explain the fact that third-row atoms such as phosphorus and sulfur can form more than four covalent bonds, whereas second-row atoms such as nitrogen and oxygen are strictly limited by the octet rule.
500px , center , 5 resonance structures of phosphorus pentafluoride
However other models describe the bonding using only s and p orbitals in agreement with the octet rule. A valence bond description of PF5 uses resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
between different PF4+ F− structures, so that each F is bonded by a covalent bond in four structures and an ionic bond in one structure. Each resonance structure has eight valence electrons on P. A molecular orbital theory description considers the highest occupied molecular orbital to be a non-bonding orbital localized on the five fluorine atoms, in addition to four occupied bonding orbitals, so again there are only eight valence electrons on the phosphorus. The validity of the octet rule for hypervalent molecules is further supported by ab initio molecular orbital calculations, which show that the contribution of d functions to the bonding orbitals is small.
Nevertheless, for historical reasons, structures implying more than eight electrons around elements like P, S, Se, or I are still common in textbooks and research articles. In spite of the unimportance of d shell expansion in chemical bonding, this practice allows structures to be shown without using a large number of formal charges or using partial bonds and is recommended by the IUPAC as a convenient formalism in preference to depictions that better reflect the bonding. On the other hand, showing more than eight electrons around Be, B, C, N, O, or F (or more than two around H, He, or Li) is considered an error by most authorities.
Other rules
The octet rule is only applicable tomain-group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
s. Other elements follow other electron counting rules as their valence electron configurations are different from main-group elements. These other rules are shown below:
* The duet rule or duplet rule of the first shell applies to H, He and Li—the noble gas helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
has two electrons in its outer shell, which is very stable. (Since there is no 1''p'' subshell, 1''s'' is followed immediately by 2''s'', and thus shell 1 can only have at most 2 valence electrons). Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
only needs one additional electron to attain this stable configuration, while lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
needs to lose one.
* For transition metals, molecules tend to obey the 18-electron rule which corresponds to the utilization of valence ''d'', ''s'' and ''p'' orbitals to form bonding and non-bonding orbitals. However, unlike the octet rule for main-group elements, transition metals do not strictly obey the 18-electron rule and the valence electron count can vary between 12 and 18.
See also
* Lewis structure * Electron countingReferences
{{Electron configuration navbox Chemical bonding Rules of thumb