|
Triphenylcarbenium
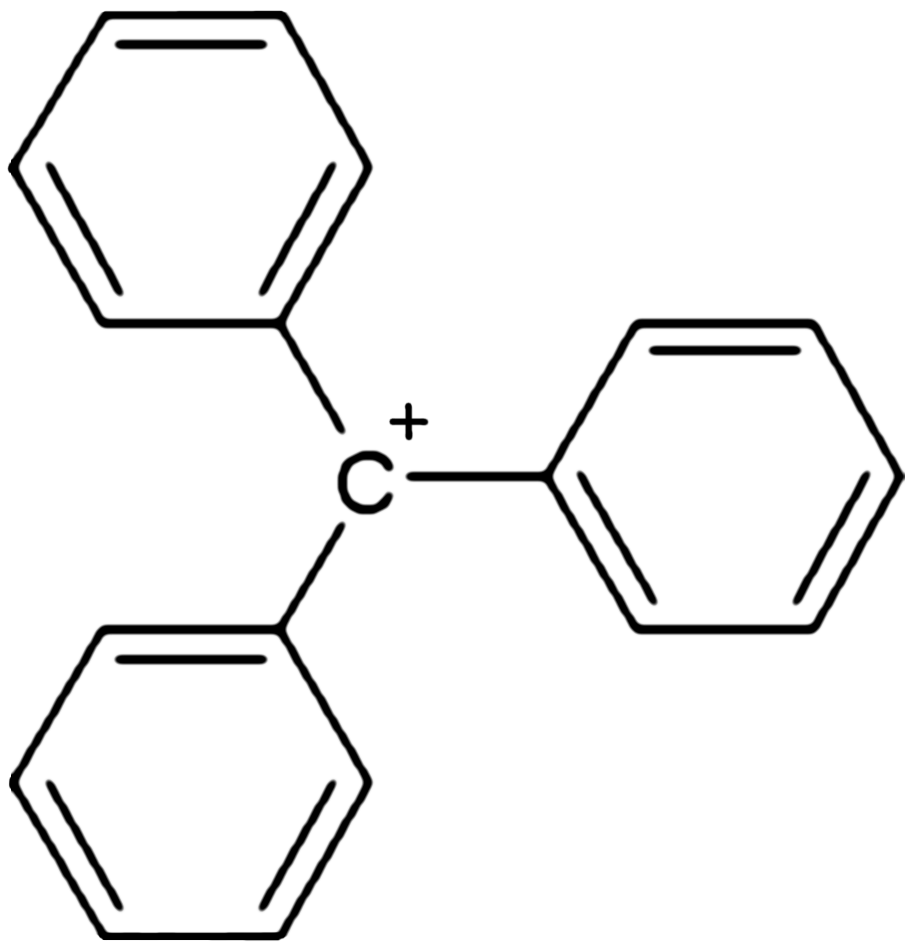
In chemistry, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon atom with a positive charge connected to three phenyl groups. It is a charged version of the triphenylmethyl radical . The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride that do not contain the cation. Triphenylcarbenium is a relatively stable carbenium ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom). Derivatives The cation exists in important chemical reagents and catalysts such as triphenylmethyl hexafluorophosphate . Related salts are known with diverse anions including tetrafluoroborate (), hexachloroantimonate (), and perchlorate (). This and other simil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenium
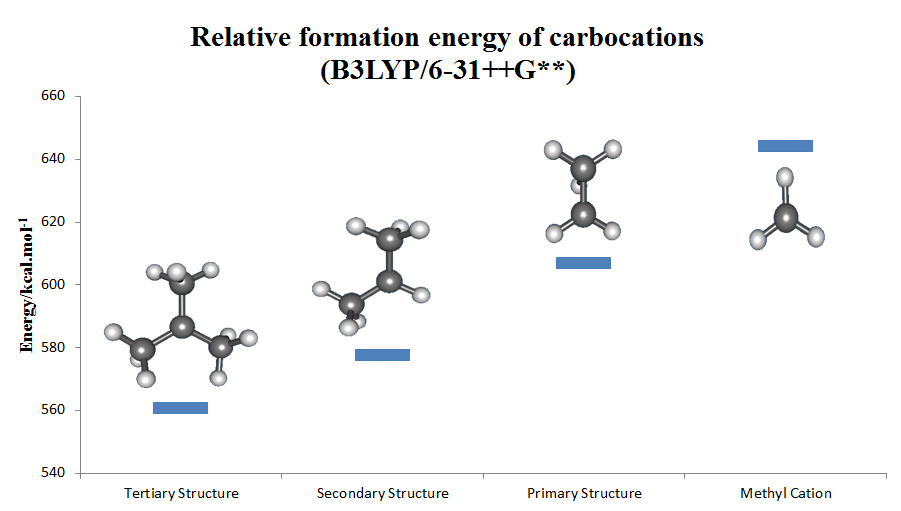
The carbenium ion is a kind of positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the carbonium ions with the formula R5+. In carbenium ions charge is localized. They are isoelectronic with monoboranes such as B(CH3)3. Nomenclature Reactivity Carbenium ions are generally highly reactive due to having an incomplete octet of electrons; however, certain carbenium ions, such as the tropylium ion, are relatively stable due to the positive charge being delocalised between the carbon atoms.(It can even exist stably in aqueous solution.) Rearrangements Carbenium ions sometimes rearrange readily. For example, when pentan-3-ol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylcarbenium
In chemistry, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon atom with a positive charge connected to three phenyl groups. It is a charged version of the triphenylmethyl radical . The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride that do not contain the cation. Triphenylcarbenium is a relatively stable carbenium ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom). Derivatives The cation exists in important chemical reagents and catalysts such as triphenylmethyl hexafluorophosphate . Related salts are known with diverse anions including tetrafluoroborate (), hexachloroantimonate (), and perchlorate (). This and other simil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethyl Hexafluorophosphate
Triphenylmethyl hexafluorophosphate (also triphenylcarbenium hexafluorophosphate, trityl hexafluorophosphate, or tritylium hexafluorophosphate) is an organic salt with the formula , consisting of the triphenylcarbenium cation and the hexafluorophosphate anion . Triphenylmethyl hexafluorophosphate is a brown powder that hydrolyzes readily to triphenylmethanol. It is used as a catalyst and reagent in organic syntheses. Preparation Triphenylmethyl hexafluorophosphate can be prepared by combining silver hexafluorophosphate with triphenylmethyl chloride: : A second method involves protonolysis of triphenylmethanol: : Structure and reactions Triphenylmethyl hexafluorophosphate readily hydrolyzes, in a reaction that is the reverse of one of its syntheses: : Triphenylmethyl hexafluorophosphate has been used for abstracting hydride () from organic compounds. Treatment of metal-alkene and diene complexes one can generate allyl and pentadienyl complexes, respectively. Tripheny ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The simplest aryl group is phenyl, which is made up of a benzene ring with one of its hydrogen atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethane
Triphenylmethane or triphenyl methane (sometimes also known as Tritan), is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical). Preparation Triphenylmethane was first synthesized in 1872 by the German chemist August Kekulé and his Dutch student Antoine Paul Nicolas Franchimont (1844–1919) by heating diphenylmercury (Hg(C6H5)2, ''Quecksilberdiphenyl'') with benzal chloride (C6H5CHCl2, ''Benzylenchlorid''). Triphenylmethane can be synthesized by Friedel–Crafts reaction from benzene and chloroform with aluminium chloride catalyst: :3 C6H6 + CHCl3 → Ph3CH + 3 HCl Alternatively, benzene may re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pararosaniline
Pararosaniline, pararosaniline free base, Basic Red 9, or C.I. 42500 is an organic compound with the formula . It is the free base form of pararosaniline hydrochloride, , a magenta solid with a variety of uses as a dye. It is one of the four components of basic fuchsine. It is structurally related to other triarylmethane dyes called methyl violets (e.g. crystal violet) which feature methyl groups on nitrogen. It is prepared by the condensation of aniline and . Alternatively, it arises from the oxidation of 4,4'-bis(aminophenyl)methane in the presence of aniline. Uses *It is used to dye polyacrylonitrile fibers. *It is used to detect sulfur dioxide. *Pararosaniline is used as a colorimetric test for aldehydes, in the Schiff test. It is the only basic fuchsine component suitable for making the aldehyde-fuchsine stain for pancreatic islet beta cells. *It has use as an antischistosomal. Related compounds * 4,4'-Thiodianiline * 4,4'-Methylenedianiline * 4,4'-Oxydianiline * Dapso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New Fuchsine
New fuchsine is an organic compound with the formula H2N(CH3)C6H3)3Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes. The other is pararosaniline. It is prepared by condensation of ortho-toluidine with formaldehyde. This process initially gives the benzhydrol 4,4'-bis(dimethylamino)benzhydrol, which is further condensed to give the leuco (colorless) tertiary alcohol [(H2N(CH3)C6H3)3COH, which is oxidized in acid to give the dye. Use as dye and stain New fuchsine is used to dye polyacrylonitrile, paper, and leather. In biology, it can be used for staining (biology), staining acid-fast organisms, e.g. by Ziehl–Neelsen stain, and for making Schiff's reagent. As a primary amine, the dye can be diazotized in the laboratory, and the resulting diazonium salt used as a trapping agent in enzyme histochemistry.Lojda Z, Gossrau R, Schiebler TH (1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Violet
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triphenylmethane, triarylmethane dye used as a histological stain and in Gram staining, Gram's method of classifying bacteria. Crystal violet has antibacterial, Antifungal medication, antifungal, and anthelmintic (Anthelmintic, vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization. The name ''gentian violet'' was originally used for a mixture of methyl pararosaniline dyes (methyl violet), but is now often considered a synonym for ''crystal violet''. The name refers to its colour, being like that of the petals of certain Gentiana, gentian flowers; it is not made from gentians or list of plants known as violet, violets. Production A number of possible routes can be used to prepare crystal violet. The original procedur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiley-VCH
Wiley-VCH is a German publisher owned by John Wiley & Sons. It was founded in 1921 as Verlag Chemie (meaning "Chemistry Press": VCH stands for ''Verlag Chemie'') by two German learned societies A learned society ( ; also scholarly, intellectual, or academic society) is an organization that exists to promote an academic discipline, profession, or a group of related disciplines such as the arts and sciences. Membership may be open to al .... Later, it was merged into the German Chemical Society (GDCh). In 1991, VCH acquired Akademie Verlag. It has been owned by John Wiley & Sons since 1996. The humanities section of Akademie Verlag and the Akademie brand were sold in 1997 to R. Oldenbourg Verlag, while VCH retained the natural sciences catalog. References External links * Wiley (publisher) Publishing companies of Germany Publishing companies established in 1921 Weinheim German companies established in 1921 {{publish-company-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann's Encyclopedia Of Industrial Chemistry
''Ullmann's Encyclopedia of Industrial Chemistry'' is a major reference work related to Chemical industry, industrial chemistry by chemist Fritz Ullmann, first published in 1914, and exclusively in German as "Enzyklopädie der Technischen Chemie" until 1984. History Ullmann's Encyclopedia of Industrial Chemistry is a major reference work related to industrial chemistry by chemist Fritz Ullmann. Its first edition was published in German by Fritz Ullmann in 1914. The fourth edition, published 1972 to 1984, already contained 25 volumes. The fifth edition, published 1985 to 1996, was the first version available in English. In 1997, the first online version was published. The year 2014 marked its Centennial, centenary. Ullmann's Encyclopedia was in its seventh edition, in 40 volumes, including one index volume and more than 1,050 articles (200 more than the sixth edition), approximately 30,000 pages, 22,000 images, 8,000 tables, 19,000 references and 85,000 indices. Editions * 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triarylmethane Dye
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes. Families Triarylmethane dyes can be grouped into families according to the nature of the substituents on the aryl groups. In some cases, the anions associated with the cationic dyes (say crystal violet) vary even though the name of the dye does not. Often it is shown as chloride. Methyl violet dyes Methyl violet dyes have dimethylamino groups at the ''p''-positions of two aryl groups. Image:Methyl Violet 2B.svg, Methyl violet 2B Image:Methyl Violet 6B.svg, Methyl violet 6B Image:Kristallviolett.svg, Methyl violet 10B Fuchsine dyes Fuchsine dyes have primary or secondary amines (NH2 or NHMe) functional groups at the ''p''-positions of each aryl group. File:Pararosaniline.png, Pararosaniline File:Rosaniline hydrochloride.svg, Fuchsine (hydrochloride salt) Neofuchsin.svg, New fuchsine (As chlorid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |