light wave on:
[Wikipedia]
[Google]
[Amazon]
 In
In

 Electric and magnetic fields obey the properties of superposition. Thus, a field due to any particular particle or time-varying electric or magnetic field contributes to the fields present in the same space due to other causes. Further, as they are vector fields, all magnetic and electric field vectors add together according to vector addition. For example, in optics two or more coherent light waves may interact and by constructive or destructive interference yield a resultant irradiance deviating from the sum of the component irradiances of the individual light waves. The electromagnetic fields of light are not affected by traveling through static electric or magnetic fields in a linear medium such as a vacuum. However, in nonlinear media, such as some
Electric and magnetic fields obey the properties of superposition. Thus, a field due to any particular particle or time-varying electric or magnetic field contributes to the fields present in the same space due to other causes. Further, as they are vector fields, all magnetic and electric field vectors add together according to vector addition. For example, in optics two or more coherent light waves may interact and by constructive or destructive interference yield a resultant irradiance deviating from the sum of the component irradiances of the individual light waves. The electromagnetic fields of light are not affected by traveling through static electric or magnetic fields in a linear medium such as a vacuum. However, in nonlinear media, such as some
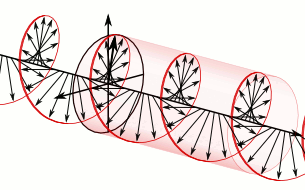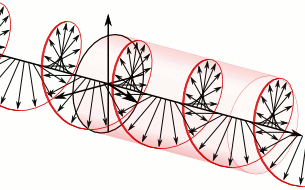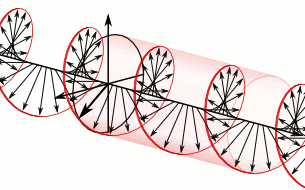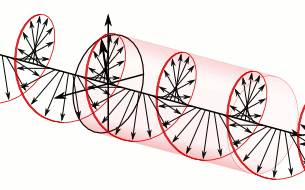 In homogeneous, isotropic media, electromagnetic radiation is a transverse wave, meaning that its oscillations are perpendicular to the direction of energy transfer and travel. It comes from the following equations:These equations predicate that any electromagnetic wave must be a transverse wave, where the electric field and the magnetic field are both perpendicular to the direction of wave propagation. The electric and magnetic parts of the field in an electromagnetic wave stand in a fixed ratio of strengths to satisfy the two
In homogeneous, isotropic media, electromagnetic radiation is a transverse wave, meaning that its oscillations are perpendicular to the direction of energy transfer and travel. It comes from the following equations:These equations predicate that any electromagnetic wave must be a transverse wave, where the electric field and the magnetic field are both perpendicular to the direction of wave propagation. The electric and magnetic parts of the field in an electromagnetic wave stand in a fixed ratio of strengths to satisfy the two
 Maxwell's equations established that some charges and currents (''sources'') produce local electromagnetic fields near them that do not radiate. Currents directly produce magnetic fields, but such fields of a magnetic-dipole–type that dies out with distance from the current. In a similar manner, moving charges pushed apart in a conductor by a changing electrical potential (such as in an antenna) produce an electric-dipole–type electrical field, but this also declines with distance. These fields make up the '' near'' field. Neither of these behaviours is responsible for EM radiation. Instead, they only efficiently transfer energy to a receiver very close to the source, such as inside a transformer. The near field has strong effects on its source, with any energy withdrawn by a receiver causing increased ''load'' (decreased
Maxwell's equations established that some charges and currents (''sources'') produce local electromagnetic fields near them that do not radiate. Currents directly produce magnetic fields, but such fields of a magnetic-dipole–type that dies out with distance from the current. In a similar manner, moving charges pushed apart in a conductor by a changing electrical potential (such as in an antenna) produce an electric-dipole–type electrical field, but this also declines with distance. These fields make up the '' near'' field. Neither of these behaviours is responsible for EM radiation. Instead, they only efficiently transfer energy to a receiver very close to the source, such as inside a transformer. The near field has strong effects on its source, with any energy withdrawn by a receiver causing increased ''load'' (decreased
 In 1862–64
In 1862–64
The Growth of Physical Science
Cambridge University Press

 EM radiation (the designation 'radiation' excludes static electric and magnetic and near fields) is classified by wavelength into
EM radiation (the designation 'radiation' excludes static electric and magnetic and near fields) is classified by wavelength into
 Most UV and X-rays are blocked by absorption first from molecular
Most UV and X-rays are blocked by absorption first from molecular
The Feynman Lectures on Physics Vol. I Ch. 28: Electromagnetic Radiation
*
''Electromagnetic Waves from Maxwell's Equations''
o
Project PHYSNET
{{Portal bar, Physics Heinrich Hertz Radiation
physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
, electromagnetic radiation (EMR) is a self-propagating wave
In physics, mathematics, engineering, and related fields, a wave is a propagating dynamic disturbance (change from List of types of equilibrium, equilibrium) of one or more quantities. ''Periodic waves'' oscillate repeatedly about an equilibrium ...
of the electromagnetic field that carries momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
and radiant energy
In physics, and in particular as measured by radiometry, radiant energy is the energy of electromagnetic radiation, electromagnetic and gravitational radiation. As energy, its SI unit is the joule (J). The quantity of radiant energy may be calcul ...
through space. It encompasses a broad spectrum, classified by frequency
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
or its inverse, wavelength, ranging from radio waves
Radio waves (formerly called Hertzian waves) are a type of electromagnetic radiation with the lowest frequencies and the longest wavelengths in the electromagnetic spectrum, typically with frequencies below 300 gigahertz (GHz) and wavelengths ...
, microwaves, infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
, visible light, ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
, X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s, and gamma rays
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
. All forms of EMR travel at the speed of light in a vacuum and exhibit wave–particle duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the in ...
, behaving both as waves and as discrete particles called photons.
Electromagnetic radiation is produced by accelerating charged particles such as from the Sun and other celestial bodies or artificially generated for various applications. Its interaction with matter depends on wavelength, influencing its uses in communication, medicine, industry, and scientific research. Radio waves enable broadcasting
Broadcasting is the data distribution, distribution of sound, audio audiovisual content to dispersed audiences via a electronic medium (communication), mass communications medium, typically one using the electromagnetic spectrum (radio waves), ...
and wireless communication, infrared is used in thermal imaging, visible light is essential for vision, and higher-energy radiation, such as X-rays and gamma rays, is applied in medical imaging, cancer treatment, and industrial inspection. Exposure to high-energy radiation can pose health risks, making shielding and regulation necessary in certain applications.
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
, an alternate way of viewing EMR is that it consists of photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s, uncharged elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. The Standard Model presently recognizes seventeen distinct particles—twelve fermions and five bosons. As a c ...
s with zero rest mass which are the quanta of the electromagnetic field, responsible for all electromagnetic interactions. Quantum electrodynamics
In particle physics, quantum electrodynamics (QED) is the Theory of relativity, relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quant ...
is the theory of how EMR interacts with matter on an atomic level. Quantum effects provide additional sources of EMR, such as the transition of electrons to lower energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
s in an atom and black-body radiation.
Physics
Properties
Electromagnetic radiation is produced by accelerating charged particles and can be naturally emitted, as from the Sun and other celestial bodies, or artificially generated for various applications. The energy in electromagnetic waves is sometimes calledradiant energy
In physics, and in particular as measured by radiometry, radiant energy is the energy of electromagnetic radiation, electromagnetic and gravitational radiation. As energy, its SI unit is the joule (J). The quantity of radiant energy may be calcul ...
. The electromagnetic waves' energy does not need a propagating medium to travel through space; they move through a vacuum at the speed of light.
 Electric and magnetic fields obey the properties of superposition. Thus, a field due to any particular particle or time-varying electric or magnetic field contributes to the fields present in the same space due to other causes. Further, as they are vector fields, all magnetic and electric field vectors add together according to vector addition. For example, in optics two or more coherent light waves may interact and by constructive or destructive interference yield a resultant irradiance deviating from the sum of the component irradiances of the individual light waves. The electromagnetic fields of light are not affected by traveling through static electric or magnetic fields in a linear medium such as a vacuum. However, in nonlinear media, such as some
Electric and magnetic fields obey the properties of superposition. Thus, a field due to any particular particle or time-varying electric or magnetic field contributes to the fields present in the same space due to other causes. Further, as they are vector fields, all magnetic and electric field vectors add together according to vector addition. For example, in optics two or more coherent light waves may interact and by constructive or destructive interference yield a resultant irradiance deviating from the sum of the component irradiances of the individual light waves. The electromagnetic fields of light are not affected by traveling through static electric or magnetic fields in a linear medium such as a vacuum. However, in nonlinear media, such as some crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s, interactions can occur between light and static electric and magnetic fields—these interactions include the Faraday effect and the Kerr effect
The Kerr effect, also called the quadratic electro-optic (QEO) effect, is a change in the refractive index of a material in response to an applied electric field. The Kerr effect is distinct from the Pockels effect in that the induced index chan ...
.
In refraction
In physics, refraction is the redirection of a wave as it passes from one transmission medium, medium to another. The redirection can be caused by the wave's change in speed or by a change in the medium. Refraction of light is the most commo ...
, a wave crossing from one medium to another of different density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
alters its speed and direction upon entering the new medium. The ratio of the refractive indices of the media determines the degree of refraction, and is summarized by Snell's law. Light of composite wavelengths (natural sunlight) disperses into a visible spectrum
A spectrum (: spectra or spectrums) is a set of related ideas, objects, or properties whose features overlap such that they blend to form a continuum. The word ''spectrum'' was first used scientifically in optics to describe the rainbow of co ...
passing through a prism, because of the wavelength-dependent refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
of the prism material ( dispersion); that is, each component wave within the composite light is bent a different amount.
EM radiation exhibits both wave properties and particle
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
properties at the same time (known as wave–particle duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the in ...
). Both wave and particle characteristics have been confirmed in many experiments. Wave characteristics are more apparent when EM radiation is measured over relatively large timescales and over large distances while particle characteristics are more evident when measuring small timescales and distances. For example, when electromagnetic radiation is absorbed by matter, particle-like properties will be more obvious when the average number of photons in the cube of the relevant wavelength is much smaller than 1. It is not so difficult to experimentally observe non-uniform deposition of energy when light is absorbed, however this alone is not evidence of "particulate" behavior. Rather, it reflects the quantum nature of ''matter''. A quantum theory of the interaction between electromagnetic radiation and matter such as electrons is described by the theory of quantum electrodynamics
In particle physics, quantum electrodynamics (QED) is the Theory of relativity, relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quant ...
.
Electromagnetic waves can be polarized, reflected, refracted, or diffracted, and can interfere with each other. Some experiments display both the wave and particle natures of electromagnetic waves, such as the self-interference of a single photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
. When a low intensity light is sent through an interferometer it will detected by a photomultiplier or other sensitive detector only along one arm of the device, consistent with particle properties, and yet the accumulated effect of many such detections will be interference consistent with wave properties.
Wave model
 In homogeneous, isotropic media, electromagnetic radiation is a transverse wave, meaning that its oscillations are perpendicular to the direction of energy transfer and travel. It comes from the following equations:These equations predicate that any electromagnetic wave must be a transverse wave, where the electric field and the magnetic field are both perpendicular to the direction of wave propagation. The electric and magnetic parts of the field in an electromagnetic wave stand in a fixed ratio of strengths to satisfy the two
In homogeneous, isotropic media, electromagnetic radiation is a transverse wave, meaning that its oscillations are perpendicular to the direction of energy transfer and travel. It comes from the following equations:These equations predicate that any electromagnetic wave must be a transverse wave, where the electric field and the magnetic field are both perpendicular to the direction of wave propagation. The electric and magnetic parts of the field in an electromagnetic wave stand in a fixed ratio of strengths to satisfy the two Maxwell's equations
Maxwell's equations, or Maxwell–Heaviside equations, are a set of coupled partial differential equations that, together with the Lorentz force law, form the foundation of classical electromagnetism, classical optics, Electrical network, electr ...
that specify how one is produced from the other. In dissipation-less (lossless) media, these and fields are also in phase, with both reaching maxima and minima at the same points in space.
In the far-field EM radiation which is described by the two source-free Maxwell curl operator equations, a time-change in one type of field is proportional to the curl of the other. These derivatives require that the and fields in EMR are in phase. An important aspect of light's nature is its frequency
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
. The frequency of a wave is its rate of oscillation and is measured in hertz
The hertz (symbol: Hz) is the unit of frequency in the International System of Units (SI), often described as being equivalent to one event (or Cycle per second, cycle) per second. The hertz is an SI derived unit whose formal expression in ter ...
, the SI unit of frequency, where one hertz is equal to one oscillation per second. Light usually has multiple frequencies that sum to form the resultant wave. Different frequencies undergo different angles of refraction, a phenomenon known as dispersion.
A monochromatic wave (a wave of a single frequency) consists of successive troughs and crests, and the distance between two adjacent crests or troughs is called the wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
. Waves of the electromagnetic spectrum vary in size, from very long radio waves longer than a continent to very short gamma rays smaller than atom nuclei. Frequency is inversely proportional to wavelength, according to the equation:
:
where ''v'' is the speed of the wave ( ''c'' in a vacuum or less in other media), ''f'' is the frequency, and ''λ'' is the wavelength. As waves cross boundaries between different media, their speeds change but their frequencies remain constant.
Electromagnetic waves in free space must be solutions of Maxwell's electromagnetic wave equation. Two main classes of solutions are known, namely plane waves and spherical waves. The plane waves may be viewed as the limiting case of spherical waves at a very large (ideally infinite) distance from the source. Both types of waves can have a waveform which is an arbitrary time function (so long as it is sufficiently differentiable to conform to the wave equation). As with any time function, this can be decomposed by means of Fourier analysis into its frequency spectrum
In signal processing, the power spectrum S_(f) of a continuous time signal x(t) describes the distribution of power into frequency components f composing that signal. According to Fourier analysis, any physical signal can be decomposed int ...
, or individual sinusoidal components, each of which contains a single frequency, amplitude, and phase. Such a component wave is said to be ''monochromatic''.
Interference is the superposition of two or more waves resulting in a new wave pattern. If the fields have components in the same direction, they constructively interfere, while opposite directions cause destructive interference. Additionally, multiple polarization signals can be combined (i.e. interfered) to form new states of polarization, which is known as parallel polarization state generation.
Maxwell's equations
James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
derived a wave form of the electric and magnetic equations, thus uncovering the wave-like nature of electric
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
and magnetic fields and their symmetry
Symmetry () in everyday life refers to a sense of harmonious and beautiful proportion and balance. In mathematics, the term has a more precise definition and is usually used to refer to an object that is Invariant (mathematics), invariant und ...
. Because the speed of EM waves predicted by the wave equation coincided with the measured speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant exactly equal to ). It is exact because, by international agreement, a metre is defined as the length of the path travelled by light in vacuum during a time i ...
, Maxwell concluded that light itself is an EM wave. Maxwell's equations were confirmed by Heinrich Hertz
Heinrich Rudolf Hertz (; ; 22 February 1857 – 1 January 1894) was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwell's equations of electromagnetism.
Biography
Heinri ...
through experiments with radio waves. Out of the four equations, two of the equations that Maxwell refine were Faraday's Law of Induction and Ampère's circuital law, which he extended by adding the displacement current term to the equations himself. Maxwell thought that the displacement current, which he viewed as the motion of bound charges, gave rise to the magnetic field. The other two equations are Gauss's law and Gauss's law for magnetism.
Near and far fields
electrical reactance
In electrical circuits, reactance is the opposition presented to alternating current by inductance and capacitance. It's measured in Ohm, Ω (Ohms). Along with resistance, it is one of two elements of Electrical impedance, impedance; however, whi ...
) on the source. The near field does not propagate freely into space, carrying energy away without a distance limit, but rather oscillates, returning its energy to the transmitter if it is not absorbed by a receiver.
By contrast, the '' far'' field is composed of ''radiation'' that is free of the transmitter, in the sense that the transmitter requires the same power to send changes in the field out regardless of whether anything absorbs the signal, e.g. a radio station does not need to increase its power when more receivers use the signal. This far part of the electromagnetic field ''is'' electromagnetic radiation. The far fields propagate (radiate) without allowing the transmitter to affect them. This causes them to be independent in the sense that their existence and their energy, after they have left the transmitter, is completely independent of both transmitter and receiver. Due to conservation of energy
The law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be Conservation law, ''conserved'' over time. In the case of a Closed system#In thermodynamics, closed system, the principle s ...
, the amount of power passing through any closed surface drawn around the source is the same. The power density of EM radiation from an isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also ...
source decreases with the inverse square of the distance from the source; this is called the inverse-square law. Field intensity due to dipole parts of the near field varies according to an inverse-cube law, and thus fades with distance.
In the Liénard–Wiechert potential formulation of the electric and magnetic fields due to motion of a single particle (according to Maxwell's equations), the terms associated with acceleration of the particle are those that are responsible for the part of the field that is regarded as electromagnetic radiation. By contrast, the term associated with the changing static electric field of the particle and the magnetic term that results from the particle's uniform velocity are both associated with the near field, and do not comprise electromagnetic radiation.
Particle model and quantum theory
An anomaly arose in the late 19th century involving a contradiction between the wave theory of light and measurements of the electromagnetic spectra that were being emitted by thermal radiators known as black bodies. Physicists struggled with this problem unsuccessfully for many years, and it later became known as the ultraviolet catastrophe. In 1900,Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
developed a new theory of black-body radiation that explained the observed spectrum. Planck's theory was based on the idea that black bodies emit light (and other electromagnetic radiation) only as discrete bundles or packets of energy. These packets were called quanta. In 1905, Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
proposed that light quanta be regarded as real particles. Later the particle of light was given the name photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
, to correspond with other particles being described around this time, such as the electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
and proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
. A photon has an energy, ''E'', proportional to its frequency, ''f'', by
:
where ''h'' is the Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
, is the wavelength and ''c'' is the speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant exactly equal to ). It is exact because, by international agreement, a metre is defined as the length of the path travelled by light in vacuum during a time i ...
. This is sometimes known as the Planck–Einstein equation. In quantum theory (see first quantization) the energy of the photons is thus directly proportional to the frequency of the EMR wave. Likewise, the momentum ''p'' of a photon is also proportional to its frequency and inversely proportional to its wavelength:
:
The source of Einstein's proposal that light was composed of particles (or could act as particles in some circumstances) was an experimental anomaly not explained by the wave theory: the photoelectric effect, in which light striking a metal surface ejected electrons from the surface, causing an electric current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge c ...
to flow across an applied voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
. Experimental measurements demonstrated that the energy of individual ejected electrons was proportional to the ''frequency
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
'', rather than the '' intensity'', of the light. Furthermore, below a certain minimum frequency, which depended on the particular metal, no current would flow regardless of the intensity. These observations appeared to contradict the wave theory, and for years physicists tried to find an explanation. In 1905, Einstein explained this phenomenon by resurrecting the particle theory of light. Because of the preponderance of evidence in favor of the wave theory, however, Einstein's ideas were met initially with great skepticism among established physicists. Eventually Einstein's explanation was accepted as new particle-like behavior of light was observed, such as the Compton effect.
As a photon is absorbed by an atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
, it excites the atom, elevating an electron to a higher energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
(one that is on average farther from the nucleus). When an electron in an excited molecule or atom descends to a lower energy level, it emits a photon of light at a frequency corresponding to the energy difference. Since the energy levels of electrons in atoms are discrete, each element and each molecule emits and absorbs its own characteristic frequencies. Immediate photon emission is called fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
, a type of photoluminescence. An example is visible light emitted from fluorescent paints, in response to ultraviolet ( blacklight). Many other fluorescent emissions are known in spectral bands other than visible light. Delayed emission is called phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluor ...
.
Quantum mechanics also governs emission, which is seen when an emitting gas glows due to excitation of the atoms from any mechanism, including heat. As electrons descend to lower energy levels, a spectrum is emitted that represents the jumps between the energy levels of the electrons, but lines are seen because again emission happens only at particular energies after excitation. An example is the emission spectrum of nebula
A nebula (; or nebulas) is a distinct luminescent part of interstellar medium, which can consist of ionized, neutral, or molecular hydrogen and also cosmic dust. Nebulae are often star-forming regions, such as in the Pillars of Creation in ...
e. Rapidly moving electrons are most sharply accelerated when they encounter a region of force, so they are responsible for producing much of the highest frequency electromagnetic radiation observed in nature. These phenomena can be used to detect the composition of gases lit from behind ( absorption spectra) and for glowing gases ( emission spectra). Spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Spectro ...
(for example) determines what chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s comprise a particular star. Shifts in the frequency of the spectral lines for an element, called a redshift
In physics, a redshift is an increase in the wavelength, and corresponding decrease in the frequency and photon energy, of electromagnetic radiation (such as light). The opposite change, a decrease in wavelength and increase in frequency and e ...
, can be used to determine the star's cosmological distance.
Wave–particle duality
The modern theory that explains the nature of light includes the notion of wave–particle duality. The theory is based on the concept that every quantum entity can show wave-like or particle-like behaviors, depending on observation. The observation led to the collapse of the entity'swave function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
. If it is based on the Copenhagen interpretation, the observation does really collapse the wave function; for the many-worlds interpretation, all possible outcomes of the collapse happened in parallel universes; for the pilot wave theory, the particle behaviour is simply determined by waves. The duality nature of a real photon has been observed in the double-slit experiment.
Together, wave and particle effects fully explain the emission and absorption spectra of EM radiation. The matter-composition of the medium through which the light travels determines the nature of the absorption and emission spectrum. These bands correspond to the allowed energy levels in the atoms. Dark bands in the absorption spectrum are due to the atoms in an intervening medium between source and observer. The atoms absorb certain frequencies of the light between emitter and detector/eye, then emit them in all directions. A dark band appears to the detector, due to the radiation scattered out of the light beam. For instance, dark bands in the light emitted by a distant star
A star is a luminous spheroid of plasma (physics), plasma held together by Self-gravitation, self-gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night sk ...
are due to the atoms in the star's atmosphere.
Propagation speed
In empty space (vacuum), electromagnetic radiation travels at thespeed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant exactly equal to ). It is exact because, by international agreement, a metre is defined as the length of the path travelled by light in vacuum during a time i ...
, , 299,792,458 meters per second (approximately 186,000 miles per second). In a medium other than vacuum it travels at a lower velocity , given by a dimensionless parameter between 0 and 1 characteristic of the medium called the velocity factor or its reciprocal, the refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
:
:.
The reason for this is that in matter the electric and magnetic fields of the wave are slowed because they polarize the charged particles in the medium they pass through. The oscillating electric field causes nearby positive and negative charges in atoms to move slightly apart and together, inducing an oscillating polarization, creating an electric polarization field. The oscillating magnetic field moves nearby magnetic dipoles, inducing an oscillating magnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Accordingly, physicists and engineers usually define magnetization as the quanti ...
, creating an induced oscillating magnetic field. These induced fields, superposed on the original wave fields, slow the wave ( Ewald–Oseen extinction theorem). The amount of slowing depends on the electromagnetic properties of the medium, the electric permittivity and magnetic permeability. In the SI system of units, empty space has a vacuum permittivity of 8.854×10−12 F/m (farad
The farad (symbol: F) is the unit of electrical capacitance, the ability of a body to store an electrical charge, in the International System of Units, International System of Units (SI), equivalent to 1 coulomb per volt (C/V). It is named afte ...
s per meter) and a vacuum permeability
The vacuum magnetic permeability (variously ''vacuum permeability'', ''permeability of free space'', ''permeability of vacuum'', ''magnetic constant'') is the magnetic permeability in a classical vacuum. It is a physical constant, conventionally ...
of 1.257×10−6 H/m ( henries per meter). These universal constants determine the speed of light in a vacuum:
:
In a medium that is isotropic and linear, which means the electric polarization is proportional to the electric field and the magnetization is proportional to the magnetic field . The speed of the waves, the , and the refractive index are determined by only two parameters: the electric permittivity of the medium in farads per meter, and the magnetic permeability of the medium in henrys per meter
:
:
If the permittivity and permeability of the medium is constant for different frequency EM waves, this is called a '' non-dispersive'' medium. In this case all EM wave frequencies would travel at the same velocity, and the waveshape stays constant as it travels. However in real matter and typically vary with frequency, this is called a '' dispersive'' medium. In dispersive media different spectral bands have different propagation characteristics, and an arbitrary wave changes shape as it travels through the medium.
History of discovery
Electromagnetic radiation of wavelengths other than those of visible light were discovered in the early 19th century. The discovery ofinfrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
radiation is ascribed to astronomer William Herschel
Frederick William Herschel ( ; ; 15 November 1738 – 25 August 1822) was a German-British astronomer and composer. He frequently collaborated with his younger sister and fellow astronomer Caroline Herschel. Born in the Electorate of Hanover ...
, who published his results in 1800 before the Royal Society of London
The Royal Society, formally The Royal Society of London for Improving Natural Knowledge, is a learned society and the United Kingdom's national academy of sciences. The society fulfils a number of roles: promoting science and its benefits, r ...
. Herschel used a glass prism to refract light from the Sun and detected invisible rays that caused heating beyond the red part of the spectrum, through an increase in the temperature recorded with a thermometer
A thermometer is a device that measures temperature (the hotness or coldness of an object) or temperature gradient (the rates of change of temperature in space). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb ...
. These "calorific rays" were later termed infrared.
In 1801 German physicist Johann Wilhelm Ritter discovered ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
in an experiment similar to Herschel's, using sunlight and a glass prism. Ritter noted that invisible rays near the violet edge of a solar spectrum dispersed by a triangular prism darkened silver chloride preparations more quickly than did the nearby violet light. Ritter's experiments were an early precursor to what would become photography. Ritter noted that the ultraviolet rays (which at first were called "chemical rays") were capable of causing chemical reactions.
 In 1862–64
In 1862–64 James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
developed equations for the electromagnetic field which suggested that waves in the field would travel with a speed that was very close to the known speed of light. Maxwell therefore suggested that visible light (as well as invisible infrared and ultraviolet rays by inference) all consisted of propagating disturbances (or radiation) in the electromagnetic field. Radio waves were first produced deliberately by Heinrich Hertz
Heinrich Rudolf Hertz (; ; 22 February 1857 – 1 January 1894) was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwell's equations of electromagnetism.
Biography
Heinri ...
in 1887, using electrical circuits calculated to produce oscillations at a much lower frequency than that of visible light, following recipes for producing oscillating charges and currents suggested by Maxwell's equations. Hertz also developed ways to detect these waves, and produced and characterized what were later termed radio wave
Radio waves (formerly called Hertzian waves) are a type of electromagnetic radiation with the lowest frequencies and the longest wavelengths in the electromagnetic spectrum, typically with frequencies below 300 gigahertz (GHz) and wavelengths g ...
s and microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
s. Jeans, James (1947The Growth of Physical Science
Cambridge University Press
Wilhelm Röntgen
Wilhelm Conrad Röntgen (; 27 March 1845 – 10 February 1923), sometimes Transliteration, transliterated as Roentgen ( ), was a German physicist who produced and detected electromagnetic radiation in a wavelength range known as X-rays. As ...
discovered and named X-rays
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
. After experimenting with high voltages applied to an evacuated tube on 8 November 1895, he noticed a fluorescence on a nearby plate of coated glass. In one month, he discovered X-rays' main properties.
The last portion of the EM spectrum to be discovered was associated with radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
. Henri Becquerel found that uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
salts caused fogging of an unexposed photographic plate through a covering paper in a manner similar to X-rays, and Marie Curie discovered that only certain elements gave off these rays of energy, soon discovering the intense radiation of radium. The radiation from pitchblende
Uraninite, also known as pitchblende, is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2 but because of oxidation typically contains variable proportions of U3O8. Radioactive decay of the urani ...
was differentiated into alpha rays (alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s) and beta rays ( beta particles) by Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
through simple experimentation in 1899, but these proved to be charged particulate types of radiation. However, in 1900 the French scientist Paul Villard discovered a third neutrally charged and especially penetrating type of radiation from radium, and after he described it, Rutherford realized it must be yet a third type of radiation, which in 1903 Rutherford named gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
s.
In 1910 British physicist William Henry Bragg demonstrated that gamma rays are electromagnetic radiation, not particles, and in 1914 Rutherford and Edward Andrade measured their wavelengths, finding that they were similar to X-rays but with shorter wavelengths and higher frequency, although a 'cross-over' between X and gamma rays makes it possible to have X-rays with a higher energy (and hence shorter wavelength) than gamma rays and vice versa. The origin of the ray differentiates them, gamma rays tend to be natural phenomena originating from the unstable nucleus of an atom and X-rays are electrically generated (and hence man-made) unless they are as a result of bremsstrahlung X-radiation caused by the interaction of fast moving particles (such as beta particles) colliding with certain materials, usually of higher atomic numbers.
Electromagnetic spectrum
radio
Radio is the technology of communicating using radio waves. Radio waves are electromagnetic waves of frequency between 3 hertz (Hz) and 300 gigahertz (GHz). They are generated by an electronic device called a transmitter connec ...
, microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
, infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
, visible, ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
, X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s, and gamma rays
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
. Arbitrary electromagnetic waves can be expressed by Fourier analysis in terms of sinusoidal waves ( monochromatic radiation), which in turn can each be classified into these regions of the EMR spectrum.
For certain classes of EM waves, the waveform is most usefully treated as ''random'', and then spectral analysis must be done by slightly different mathematical techniques appropriate to random or stochastic process
In probability theory and related fields, a stochastic () or random process is a mathematical object usually defined as a family of random variables in a probability space, where the index of the family often has the interpretation of time. Sto ...
es. In such cases, the individual frequency components are represented in terms of their ''power'' content, and the phase information is not preserved. Such a representation is called the power spectral density of the random process. Random electromagnetic radiation requiring this kind of analysis is, for example, encountered in the interior of stars, and in certain other very wideband forms of radiation such as the zero-point wave field of the electromagnetic vacuum.
The behavior of EM radiation and its interaction with matter depends on its frequency, and changes qualitatively as the frequency changes. Lower frequencies have longer wavelengths, and higher frequencies have shorter wavelengths, and are associated with photons of higher energy. There is no fundamental limit known to these wavelengths or energies, at either end of the spectrum, although photons with energies near the Planck energy or exceeding it (far too high to have ever been observed) will require new physical theories to describe.
Radio and microwave
Electromagnetic radiation phenomena with wavelengths ranging from one meter to one millimeter are called microwaves; with frequencies between 300 MHz (0.3 GHz) and 300 GHz. When radio waves impinge upon a conductor, they couple to the conductor, travel along it, and induce an electric current on the conductor surface by moving the electrons of the conducting material in correlated bunches of charge. At radio and microwave frequencies, EMR interacts with matter largely as a bulk collection of charges which are spread out over large numbers of affected atoms. In electrical conductors, such induced bulk movement of charges (electric current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge c ...
s) results in absorption of the EMR, or else separations of charges that cause generation of new EMR (effective reflection of the EMR). An example is absorption or emission of radio waves by antennas, or absorption of microwaves by water or other molecules with an electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system: that is, a measure of the system's overall Chemical polarity, polarity. The International System of Units, SI unit for electric ...
, as for example inside a microwave oven
A microwave oven, or simply microwave, is an electric oven that heats and cooks food by exposing it to electromagnetic radiation in the microwave frequency range. This induces Dipole#Molecular dipoles, polar molecules in the food to rotate and ...
. These interactions produce either electric currents or heat, or both.
Infrared
Like radio and microwave, infrared (IR) is reflected by metals (and also most EMR, well into the ultraviolet range). However, unlike lower-frequency radio and microwave radiation, infrared EMR commonly interacts with dipoles present in single molecules, which change as atoms vibrate at the ends of a single chemical bond. It is consequently absorbed by a wide range of substances, causing them to increase in temperature as the vibrations dissipate as heat. The same process, run in reverse, causes bulk substances to radiate in the infrared spontaneously (see thermal radiation section below). Infrared radiation is divided into spectral subregions. While different subdivision schemes exist, the spectrum is commonly divided as near-infrared (0.75–1.4 μm), short-wavelength infrared (1.4–3 μm), mid-wavelength infrared (3–8 μm), long-wavelength infrared (8–15 μm) and far infrared (15–1000 μm). Some animals, such as for snakes, have thermo-sensitive membranes (pit organs) that can detect temperature differences, allowing them to sense infrared radiation.Visible light
Natural sources produce EM radiation across the spectrum. EM radiation with awavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
between approximately 400 nm and 700 nm is directly detected by the human eye
The human eye is a sensory organ in the visual system that reacts to light, visible light allowing eyesight. Other functions include maintaining the circadian rhythm, and Balance (ability), keeping balance.
The eye can be considered as a living ...
and perceived as visible light. Other wavelengths, especially nearby infrared (longer than 700 nm) and ultraviolet (shorter than 400 nm) are also sometimes referred to as light.
As frequency increases into the visible range, photons have enough energy to change the bond structure of some individual molecules. It is not a coincidence that this happens in the visible range, as the mechanism of vision involves the change in bonding of a single molecule, retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use ret ...
, which absorbs a single photon. The change in retinal causes a change in the shape of the rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the ''RHO'' gene and a G-protein-coupled receptor (GPCR). It is a light-sensitive receptor protein that triggers visual phototransduction in rod cells. Rhodopsin mediates dim ...
protein it is contained in, which starts the biochemical process that causes the retina
The retina (; or retinas) is the innermost, photosensitivity, light-sensitive layer of tissue (biology), tissue of the eye of most vertebrates and some Mollusca, molluscs. The optics of the eye create a focus (optics), focused two-dimensional ...
of the human eye to sense the light.
Visible light is able to affect only a tiny percentage of all molecules. Usually not in a permanent or damaging way, rather the photon excites an electron which then emits another photon when returning to its original position. This is the source of color produced by most dyes. Retinal is an exception. When a photon is absorbed, the retinal permanently changes structure from cis to trans, and requires a protein to convert it back, i.e. reset it to be able to function as a light detector again.
Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
becomes possible in this range as well, for the same reason. A single molecule of chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
is excited by a single photon. In plant tissues that conduct photosynthesis, carotenoids act to quench electronically excited chlorophyll produced by visible light in a process called non-photochemical quenching, to prevent reactions that would otherwise interfere with photosynthesis at high light levels.
Limited evidence indicate that some reactive oxygen species are created by visible light in skin, and that these may have some role in photoaging, in the same manner as ultraviolet A.
Infrared, microwaves, and radio waves are known to damage molecules and biological tissue only by bulk heating, not excitation from single photons of the radiation.
Ultraviolet
As frequency increases into the ultraviolet, photons now carry enough energy (about three electron volts or more) to excite certain doubly bonded molecules into permanent chemical rearrangement. InDNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
, this causes lasting damage. DNA is also indirectly damaged by reactive oxygen species produced by ultraviolet A (UVA), which has energy too low to damage DNA directly. This is why ultraviolet at all wavelengths can damage DNA, and is capable of causing cancer, and (for UVB) skin burns (sunburn) that are far worse than would be produced by simple heating (temperature increase) effects.
At the higher end of the ultraviolet range, the energy of photons becomes large enough to impart enough energy to electrons to cause them to be liberated from the atom, in a process called photoionisation. The energy required for this is always larger than about 10 electron volt (eV) corresponding with wavelengths smaller than 124 nm (some sources suggest a more realistic cutoff of 33 eV, which is the energy required to ionize water). This high end of the ultraviolet spectrum with energies in the approximate ionization range, is sometimes called "extreme UV". Ionizing UV is strongly filtered by the Earth's atmosphere.
X-rays and gamma rays
Electromagnetic radiation composed of photons that carry minimum-ionization energy, or more (which includes the entire spectrum with shorter wavelengths), is therefore termedionizing radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching ...
. (Many other kinds of ionizing radiation are made of non-EM particles.) Electromagnetic-type ionizing radiation extends from the extreme ultraviolet to all higher frequencies and shorter wavelengths, which means that all X-rays and gamma rays qualify. These are capable of the most severe types of molecular damage, which can happen in biology to any type of biomolecule, including mutation and cancer, and often at great depths below the skin, since the higher end of the X-ray spectrum, and all of the gamma ray spectrum, penetrate matter.
Atmosphere and magnetosphere
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, and then (for wavelengths in the upper UV) from the electronic excitation of dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (). Others are:
* Ato ...
and finally ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
at the mid-range of UV. Only 30% of the Sun's ultraviolet light reaches the ground, and almost all of this is well transmitted.
Visible light is well transmitted in air, a property known as an atmospheric window, as it is not energetic enough to excite nitrogen, oxygen, or ozone, but too energetic to excite molecular vibrational frequencies of water vapor and carbon dioxide. Absorption bands in the infrared are due to modes of vibrational excitation in water vapor. However, at energies too low to excite water vapor, the atmosphere becomes transparent again, allowing free transmission of most microwave and radio waves.
Finally, at radio wavelengths longer than 10 m or so (about 30 MHz), the air in the lower atmosphere remains transparent to radio, but plasma in certain layers of the ionosphere
The ionosphere () is the ionized part of the upper atmosphere of Earth, from about to above sea level, a region that includes the thermosphere and parts of the mesosphere and exosphere. The ionosphere is ionized by solar radiation. It plays ...
begins to interact with radio waves (see skywave
In radio communication, skywave or skip refers to the propagation of radio waves reflected or refracted back toward Earth from the ionosphere, an electrically charged layer of the upper atmosphere. Since it is not limited by the curvatur ...
). This property allows some longer wavelengths (100 m or 3 MHz) to be reflected and results in shortwave radio
Shortwave radio is radio transmission using radio frequencies in the shortwave bands (SW). There is no official definition of the band range, but it always includes all of the High frequency, high frequency band (HF), which extends from 3 to 30& ...
beyond line-of-sight. However, certain ionospheric effects begin to block incoming radiowaves from space, when their frequency is less than about 10 MHz (wavelength longer than about 30 m).
Thermal and electromagnetic radiation as a form of heat
The basic structure ofmatter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic pa ...
involves charged particles bound together. When electromagnetic radiation impinges on matter, it causes the charged particles to oscillate and gain energy. The ultimate fate of this energy depends on the context. It could be immediately re-radiated and appear as scattered, reflected, or transmitted radiation. It may get dissipated into other microscopic motions within the matter, coming to thermal equilibrium and manifesting itself as thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
, or even kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
, in the material. With a few exceptions related to high-energy photons (such as fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
, harmonic generation, photochemical reactions, the photovoltaic effect for ionizing radiations at far ultraviolet, X-ray, and gamma radiation), absorbed electromagnetic radiation simply deposits its energy by heating the material. This happens for infrared, microwave, and radio wave radiation.
Intense radio waves can thermally burn living tissue and can cook food. In addition to infrared laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
s, sufficiently intense visible and ultraviolet lasers can easily set paper afire. Ionizing radiation creates high-speed electrons in a material and breaks chemical bonds, but after these electrons collide many times with other atoms eventually most of the energy becomes thermal energy all in a tiny fraction of a second. This caveat also applies to UV, even though almost all of it is not ionizing, because UV can damage molecules due to electronic excitation, which is far greater per unit energy than heating effects.
Infrared radiation in the spectral distribution of a black body is usually considered a form of heat, since it has an equivalent temperature and is associated with an entropy change per unit of thermal energy. However, "heat" is a technical term in physics and thermodynamics and is often confused with thermal energy. Any type of electromagnetic energy can be transformed into thermal energy in interaction with matter. Thus, ''any'' electromagnetic radiation can "heat" (in the sense of increase the thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
temperature of) a material, when it is absorbed. The inverse or time-reversed process of absorption is thermal radiation. Much of the thermal energy in matter consists of random motion of charged particles, and this energy can be radiated away from the matter. The resulting radiation may subsequently be absorbed by another piece of matter, with the deposited energy heating the material.
The electromagnetic radiation in an opaque cavity at thermal equilibrium is effectively a form of thermal energy, having maximum radiation entropy.
Biological effects
Bioelectromagnetics is the study of the interactions and effects of EM radiation on living organisms. The effects of electromagnetic radiation upon living cells, including those in humans, depends upon the radiation's power and frequency. For low-frequency radiation (radio waves to near ultraviolet) the best-understood effects are those due to radiation power alone, acting through heating when radiation is absorbed. For these thermal effects, frequency is important as it affects the intensity of the radiation and penetration into the organism (for example, microwaves penetrate better than infrared). It is widely accepted that low frequency fields that are too weak to cause significant heating could not possibly have any biological effect. Some research suggests that weaker ''non-thermal'' electromagnetic fields (including weak ELF magnetic fields, although the latter does not strictly qualify as EM radiation) and modulated RF and microwave fields can have biological effects, though the significance of this is unclear. TheWorld Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
has classified radio frequency electromagnetic radiation as Group 2B—possibly carcinogenic. This group contains possible carcinogens such as lead, DDT, and styrene. At higher frequencies (some of visible and beyond), the effects of individual photons begin to become important, as these now have enough energy individually to directly or indirectly damage biological molecules. All UV frequencies have been classed as Group 1 carcinogens by the World Health Organization. Ultraviolet radiation from sun exposure is the primary cause of skin cancer.
Thus, at UV frequencies and higher, electromagnetic radiation does more damage to biological systems than simple heating predicts. This is most obvious in the "far" (or "extreme") ultraviolet. UV, with X-ray and gamma radiation, are referred to as ionizing radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching ...
due to the ability of photons of this radiation to produce ions and free radicals in materials (including living tissue). Since such radiation can severely damage life at energy levels that produce little heating, it is considered far more dangerous (in terms of damage-produced per unit of energy, or power) than the rest of the electromagnetic spectrum.
Use as a weapon
The heat ray is an application of EMR that makes use of microwave frequencies to create an unpleasant heating effect in the upper layer of the skin. A publicly known heat ray weapon called the Active Denial System was developed by the US military as an experimental weapon to deny the enemy access to an area. A death ray is a theoretical weapon that delivers heat ray based on electromagnetic energy at levels that are capable of injuring human tissue. An inventor of a death ray, Harry Grindell Matthews, claimed to have lost sight in his left eye while working on his death ray weapon based on a microwavemagnetron
The cavity magnetron is a high-power vacuum tube used in early radar systems and subsequently in microwave oven, microwave ovens and in linear particle accelerators. A cavity magnetron generates microwaves using the interaction of a stream of ...
from the 1920s (a normal microwave oven
A microwave oven, or simply microwave, is an electric oven that heats and cooks food by exposing it to electromagnetic radiation in the microwave frequency range. This induces Dipole#Molecular dipoles, polar molecules in the food to rotate and ...
creates a tissue damaging cooking effect inside the oven at around 2 kV/m).
Derivation from electromagnetic theory
Electromagnetic waves are predicted by the classical laws of electricity and magnetism, known asMaxwell's equations
Maxwell's equations, or Maxwell–Heaviside equations, are a set of coupled partial differential equations that, together with the Lorentz force law, form the foundation of classical electromagnetism, classical optics, Electrical network, electr ...
. There are nontrivial solutions of the homogeneous Maxwell's equations (without charges or currents), describing ''waves'' of changing electric and magnetic fields. Beginning with Maxwell's equations in free space
A vacuum (: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective (neuter ) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressur ...
:
where
* and are the electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
(measured in V/m or N/ C) and the magnetic field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
(measured in T or Wb/m2), respectively;
* yields the divergence
In vector calculus, divergence is a vector operator that operates on a vector field, producing a scalar field giving the rate that the vector field alters the volume in an infinitesimal neighborhood of each point. (In 2D this "volume" refers to ...
and the curl of a vector field ;
* and are partial derivatives (rate of change in time, with location fixed) of the magnetic and electric field;
* is the permeability of a vacuum (4 × 10−7 H/m), and is the permittivity
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter (epsilon), is a measure of the electric polarizability of a dielectric material. A material with high permittivity polarizes more ...
of a vacuum (8.85 × 10−12 F/m);
Besides the trivial solution , useful solutions can be derived with the following vector identity, valid for all vectors in some vector field:Taking the curl of the second Maxwell's equation () yields:
Evaluating the left hand side of () with the above identity and simplifying using (), yields:
Evaluating the right hand side of () by exchanging the sequence of derivatives and inserting the fourth yields:
Combining () and () again, gives a vector-valued differential equation for the electric field, solving the homogeneous Maxwell's equations:
Taking the curl of the fourth Maxwell's equation () results in a similar differential equation for a magnetic field solving the homogeneous Maxwell's equations:
Both differential equations have the form of the general wave equation for waves propagating with speed where is a function of time and location, which gives the amplitude of the wave at some time at a certain location:This is also written as:
where denotes the so-called d'Alembert operator, which in Cartesian coordinates is given as:
Comparing the terms for the speed of propagation, yields in the case of the electric and magnetic fields:
This is the speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant exactly equal to ). It is exact because, by international agreement, a metre is defined as the length of the path travelled by light in vacuum during a time i ...
in vacuum. Thus Maxwell's equations connect the vacuum permittivity , the vacuum permeability
The vacuum magnetic permeability (variously ''vacuum permeability'', ''permeability of free space'', ''permeability of vacuum'', ''magnetic constant'') is the magnetic permeability in a classical vacuum. It is a physical constant, conventionally ...
, and the speed of light, ''c''0, via the above equation. This relationship had been discovered by Wilhelm Eduard Weber and Rudolf Kohlrausch prior to the development of Maxwell's electrodynamics, however Maxwell was the first to produce a field theory consistent with waves traveling at the speed of light.
These are only two equations versus the original four, so more information pertains to these waves hidden within Maxwell's equations. A generic vector wave for the electric field has the form
Here, is a constant vector, is any second differentiable function, is a unit vector in the direction of propagation, and is a position vector. is a generic solution to the wave equation. In other words,
for a generic wave traveling in the direction.
From the first of Maxwell's equations, we getThus,which implies that the electric field is orthogonal to the direction the wave propagates. The second of Maxwell's equations yields the magnetic field, namely,Thus,
The remaining equations will be satisfied by this choice of .
The electric and magnetic field waves in the far-field travel at the speed of light. They have a special restricted orientation and proportional magnitudes, , which can be seen immediately from the Poynting vector. The electric field, magnetic field, and direction of wave propagation are all orthogonal, and the wave propagates in the same direction as . Also E and B far-fields in free space, which as wave solutions depend primarily on these two Maxwell's equations to remain in phase with each other. This is guaranteed since the generic wave solution is first order in both space and time, and the curl operator on one side of these equations results in first-order spatial derivatives of the wave solution, while the time-derivative on the other side of the equations, which gives the other field, is first-order in time, resulting in the same phase shift for both fields in each mathematical operation.
From the viewpoint of an electromagnetic wave traveling forward, the electric field might be oscillating up and down, while the magnetic field oscillates right and left. This picture can be rotated with the electric field oscillating right and left and the magnetic field oscillating down and up. This is a different solution that is traveling in the same direction. This arbitrariness in the orientation with respect to propagation direction is known as polarization. On a quantum level, it is described as photon polarization. The direction of the polarization is defined as the direction of the electric field.
More general forms of the second-order wave equations given above are available, allowing for both non-vacuum propagation media and sources. Many competing derivations exist, all with varying levels of approximation and intended applications. One very general example is a form of the electric field equation,
which was factorized into a pair of explicitly directional wave equations, and then efficiently reduced into a single uni-directional wave equation by means of a simple slow-evolution approximation.
See also
*Antenna measurement
Antenna measurement techniques refer to the testing of antenna (radio), antennas to ensure that they meet specifications or simply to characterize them. Typical antenna parameters include Antenna gain, gain, Antenna bandwidth, bandwidth, radiation ...
* Bioelectromagnetics
* Bolometer
A bolometer is a device for measuring radiant heat by means of a material having a temperature-dependent electrical resistance. It was invented in 1878 by the American astronomer Samuel Pierpont Langley.
Principle of operation
A bolometer ...
* CONELRAD
* Electromagnetic pulse
An electromagnetic pulse (EMP), also referred to as a transient electromagnetic disturbance (TED), is a brief burst of electromagnetic energy. The origin of an EMP can be natural or artificial, and can occur as an electromagnetic field, as an ...
* Electromagnetic radiation and health
* Evanescent wave coupling
* Finite-difference time-domain method
* Gravitational wave
Gravitational waves are oscillations of the gravitational field that Wave propagation, travel through space at the speed of light; they are generated by the relative motion of gravity, gravitating masses. They were proposed by Oliver Heaviside i ...
* Helicon
* Impedance of free space
* Radiation reaction
* Health effects of sunlight exposure
* Sinusoidal plane-wave solutions of the electromagnetic wave equation
References
*Further reading
* * * * * *External links
The Feynman Lectures on Physics Vol. I Ch. 28: Electromagnetic Radiation
*
''Electromagnetic Waves from Maxwell's Equations''
o
Project PHYSNET
{{Portal bar, Physics Heinrich Hertz Radiation