Lead () is a
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has
symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Pb (from
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
) and
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
82. It is a
heavy metal that is
denser than most common materials. Lead is
soft and
malleable
Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversi ...
, and also has a relatively low
melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
. When freshly cut, lead is a shiny gray with a hint of blue. It
tarnish
Tarnish is a thin layer of corrosion that forms over copper, brass, aluminum, magnesium, neodymium and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in ...
es to a dull gray color when exposed to air. Lead has the highest atomic number of any
stable element and three of its
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s are endpoints of major nuclear
decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
s of heavier elements.
Lead is a relatively unreactive
post-transition metal
The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metal ...
. Its weak metallic character is illustrated by its
amphoteric
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
Etymology and terminology
Amphoteric is d ...
nature; lead and
lead oxide
Lead oxides are a group of inorganic compounds with formulas including lead (Pb) and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), gr ...
s react with
acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Br├ĖnstedŌĆōLowry acidŌĆōbase theory, Br├ĖnstedŌĆōLowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s and
bases, and it tends to form
covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s.
Compounds of lead
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive stru ...
are usually found in the +2
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
rather than the +4 state common with lighter members of the
carbon group
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Ch ...
. Exceptions are mostly limited to
organolead compounds. Like the lighter members of the group, lead tends to
bond with itself; it can form chains and polyhedral structures.
Since lead is easily extracted from its
ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically including metals, concentrated above background levels, and that is economically viable to mine and process. The grade of ore refers to the concentration ...
s, prehistoric people in the Near East
were aware of it.
Galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crysta ...
is a principal ore of lead which often bears silver. Interest in silver helped initiate widespread extraction and use of lead in
ancient Rome
In modern historiography, ancient Rome is the Roman people, Roman civilisation from the founding of Rome, founding of the Italian city of Rome in the 8th century BC to the Fall of the Western Roman Empire, collapse of the Western Roman Em ...
. Lead production declined after the
fall of Rome
The fall of the Western Roman Empire, also called the fall of the Roman Empire or the fall of Rome, was the loss of central political control in the Western Roman Empire, a process in which the Empire failed to enforce its rule, and its vast ...
and did not reach comparable levels until the
Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
. Lead played a crucial role in the development of the
printing press
A printing press is a mechanical device for applying pressure to an inked surface resting upon a printing, print medium (such as paper or cloth), thereby transferring the ink. It marked a dramatic improvement on earlier printing methods in whi ...
, as
movable type
Movable type (US English; moveable type in British English) is the system and technology of printing and typography that uses movable Sort (typesetting), components to reproduce the elements of a document (usually individual alphanumeric charac ...
could be relatively easily cast from lead alloys. In 2014, the annual global production of lead was about ten million tonnes, over half of which was from recycling. Lead's high density, low melting point,
ductility
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic def ...
and relative inertness to
oxidation
Redox ( , , reductionŌĆōoxidation or oxidationŌĆōreduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
make it useful. These properties, combined with its relative abundance and low cost, resulted in its extensive use in
construction
Construction are processes involved in delivering buildings, infrastructure, industrial facilities, and associated activities through to the end of their life. It typically starts with planning, financing, and design that continues until the a ...
,
plumbing
Plumbing is any system that conveys fluids for a wide range of applications. Plumbing uses piping, pipes, valves, piping and plumbing fitting, plumbing fixtures, Storage tank, tanks, and other apparatuses to convey fluids. HVAC, Heating and co ...
,
batteries,
bullets
A bullet is a Kinetic energy weapon, kinetic projectile, a component of firearm ammunition that is Shooting, shot from a gun barrel. They are made of a variety of materials, such as copper, lead, steel, polymer, rubber and even wax; and are made ...
,
shots,
weights,
solder
Solder (; North American English, NA: ) is a fusible alloy, fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces aft ...
s,
pewter
Pewter () is a malleable metal alloy consisting of tin (85ŌĆō99%), antimony (approximately 5ŌĆō10%), copper (2%), bismuth, and sometimes silver. In the past, it was an alloy of tin and lead, but most modern pewter, in order to prevent lead poi ...
s,
fusible alloy
A fusible alloy is a metal alloy capable of being easily fused, i.e. easily meltable, at relatively low temperatures. Fusible alloys are commonly, but not necessarily, eutectic alloys.
Sometimes the term "fusible alloy" is used to describe alloy ...
s,
lead paint
Lead paint or lead-based paint is paint containing lead. As pigment, lead(II) chromate (, "chrome yellow"), lead(II,IV) oxide, (, "red lead"), and lead(II) carbonate (, "white lead") are the most common forms.. Lead is added to paint to acceler ...
s,
leaded gasoline
Gasoline (North American English) or petrol (Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When formulate ...
, and
radiation shielding.
Lead is a
neurotoxin
Neurotoxins are toxins that are destructive to nervous tissue, nerve tissue (causing neurotoxicity). Neurotoxins are an extensive class of exogenous chemical neurological insult (medical), insultsSpencer 2000 that can adversely affect function ...
that accumulates in soft tissues and bones. It damages the nervous system and interferes with the function of biological
enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s, causing
neurological disorder
Neurological disorders represent a complex array of medical conditions that fundamentally disrupt the functioning of the nervous system. These disorders affect the brain, spinal cord, and nerve networks, presenting unique diagnosis, treatment, and ...
s ranging from behavioral problems to brain damage, and also affects general health, cardiovascular, and renal systems.
Lead's toxicity was first documented by ancient Greek and Roman writers, who noted some of the symptoms of
lead poisoning
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, infertility, numbness and paresthesia, t ...
, but became widely recognized in Europe in the late 19th century.
Physical properties
Atomic
A lead
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
has 82
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, arranged in an
electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
of [
Xe]4f
145d
106s
26p
2. The sum of lead's first and second
ionization energies
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as
:X(g) ...
ŌĆöthe total energy required to remove the two 6p electronsŌĆöis close to that of
tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
, lead's upper neighbor in the
carbon group
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Ch ...
. This is unusual; ionization energies generally fall going down a group, as an element's outer electrons become more distant from the
nucleus
Nucleus (: nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucleu ...
, and more
shielded by smaller orbitals. The sum of the first four ionization energies of lead exceeds that of tin, contrary to what
periodic trends
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain Chemical element, elements when grouped by period (periodic table), period and/or Group (periodic table), group. They w ...
would predict. This is explained by
relativistic effects, which become significant in heavier atoms, which contract s and p orbitals such that lead's 6s electrons have larger binding energies than its 5s electrons. A consequence is the so-called
inert pair effect
The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of o ...
: the 6s electrons of lead become reluctant to participate in bonding, stabilising the +2
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
and making the distance between nearest atoms in
crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
lead unusually long.
Lead's lighter carbon group
congeners form stable or metastable
allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
with the tetrahedrally coordinated and
covalently bonded
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
diamond cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, in ...
structure. The energy levels of their outer
s- and
p-orbitals are close enough to allow mixing into four
hybrid
Hybrid may refer to:
Science
* Hybrid (biology), an offspring resulting from cross-breeding
** Hybrid grape, grape varieties produced by cross-breeding two ''Vitis'' species
** Hybridity, the property of a hybrid plant which is a union of two diff ...
sp
3 orbitals. In lead, the inert pair effect increases the separation between its s- and p-orbitals, and the gap cannot be overcome by the energy that would be released by extra bonds following hybridization. Rather than having a diamond cubic structure, lead forms
metallic bonds in which only the p-electrons are delocalized and shared between the Pb
2+ ions. Lead consequently has a
face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
structure like the similarly sized
divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
metals
calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
and
strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
.
Bulk
Pure lead has a bright, shiny gray appearance with a hint of blue. It tarnishes on contact with moist air and takes on a dull appearance, the hue of which depends on the prevailing conditions. Characteristic properties of lead include high
density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''Žü'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
, malleability, ductility, and high resistance to
corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
due to
passivation.
Lead's close-packed face-centered cubic structure and high atomic weight result in a density of 11.34 g/cm
3, which is greater than that of common metals such as
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
(7.87 g/cm
3),
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
(8.93 g/cm
3), and
zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
(7.14 g/cm
3). This density is the origin of the idiom ''to go over like a lead balloon''. Some rarer metals are denser:
tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
and
gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
are both at 19.3 g/cm
3, and
osmium
Osmium () is a chemical element; it has Symbol (chemistry), symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a Abundance of elements in Earth's crust, trace element in a ...
ŌĆöthe densest metal knownŌĆöhas a density of 22.59 g/cm
3, almost twice that of lead.
Lead is a very soft metal with a
Mohs hardness
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fair ...
of 1.5; it can be scratched with a fingernail. It is quite malleable and somewhat ductile. The
bulk modulus
The bulk modulus (K or B or k) of a substance is a measure of the resistance of a substance to bulk compression. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume.
Other mo ...
of leadŌĆöa measure of its ease of compressibilityŌĆöis 45.8
GPa
Grading in education is the application of standardized measurements to evaluate different levels of student achievement in a course. Grades can be expressed as letters (usually A to F), as a range (for example, 1 to 6), percentages, or as num ...
. In comparison, that of
aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
is 75.2 GPa; copper 137.8 GPa; and
mild steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
160ŌĆō169 GPa. Lead's
tensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
, at 12ŌĆō17 MPa, is low (that of aluminium is 6 times higher, copper 10 times, and mild steel 15 times higher); it can be strengthened by adding small amounts of copper or
antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
.

The melting point of leadŌĆöat 327.5 ┬░C (621.5 ┬░F)ŌĆöis very low compared to most metals. Its
boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of 1749 ┬░C (3180 ┬░F) is the lowest among the carbon-group elements. The
electrical resistivity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
of lead at 20 ┬░C is 192
nanoohm-meters, almost an
order of magnitude
In a ratio scale based on powers of ten, the order of magnitude is a measure of the nearness of two figures. Two numbers are "within an order of magnitude" of each other if their ratio is between 1/10 and 10. In other words, the two numbers are ...
higher than those of other industrial metals (copper at ; gold ; and aluminium at ). Lead is a
superconductor at temperatures lower than 7.19
K; this is the highest
critical temperature
Critical or Critically may refer to:
*Critical, or critical but stable, medical states
**Critical, or intensive care medicine
*Critical juncture, a discontinuous change studied in the social sciences.
*Critical Software, a company specializing in ...
of all
type-I superconductor
The interior of a bulk superconductor cannot be penetrated by a weak magnetic field, a phenomenon known as the Meissner effect. When the applied magnetic field becomes too large, superconductivity breaks down. Superconductors can be divided into ...
s and the third highest of the elemental superconductors.
Isotopes
Natural lead consists of four stable
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s with mass numbers of 204, 206, 207, and 208, and traces of six short-lived radioisotopes with mass numbers 209ŌĆō214 inclusive. The high number of isotopes is consistent with lead's
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
being even. Lead has a
magic number of protons (82), for which the
nuclear shell model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model utilizes the Pauli exclusion principle to model the structure of atomic nuclei in terms of energy levels. The first shell model was proposed by Dmitri Ivanenk ...
accurately predicts an especially stable nucleus. Lead-208 has 126 neutrons, another magic number, which may explain why lead-208 is extraordinarily stable.
With its high atomic number, lead is the heaviest element whose natural isotopes are regarded as stable; lead-208 is the heaviest stable nucleus. (This distinction formerly fell to
bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
, with an atomic number of 83, until its only
primordial isotope
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
, bismuth-209, was found in 2003 to decay very slowly.) The four stable isotopes of lead could theoretically undergo
alpha decay
Alpha decay or ╬▒-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
to isotopes of
mercury with a release of energy, but this has not been observed for any of them; their predicted half-lives range from 10
35 to 10
189 years (at least 10
25 times the current age of the universe).

Three of the stable isotopes are found in three of the four major
decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
s: lead-206, lead-207, and lead-208 are the final decay products of
uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
,
uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
, and
thorium-232
Thorium-232 () is the main naturally occurring isotope of thorium, with a relative abundance of 99.98%. It has a half life of 14.05 billion years, which makes it the longest-lived isotope of thorium. It decays by alpha decay to radium-228; its de ...
, respectively. These decay chains are called the
uranium chain, the
actinium chain, and the
thorium chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
. Their isotopic concentrations in a natural rock sample depends greatly on the presence of these three parent uranium and thorium isotopes. For example, the relative abundance of lead-208 can range from 52% in normal samples to 90% in thorium ores; for this reason, the standard atomic weight of lead is given to only one decimal place. As time passes, the ratio of lead-206 and lead-207 to lead-204 increases, since the former two are supplemented by radioactive decay of heavier elements while the latter is not; this allows for
leadŌĆōlead dating
LeadŌĆōlead dating is a method for dating geological samples, normally based on 'whole-rock' samples of material such as granite. For most dating requirements it has been superseded by uraniumŌĆōlead dating (UŌĆōPb dating), but in certain special ...
. As uranium decays into lead, their relative amounts change; this is the basis for
uraniumŌĆōlead dating
UraniumŌĆōlead dating, abbreviated UŌĆōPb dating, is one of the oldest and most refined of the radiometric dating schemes. It can be used to date rocks that formed and crystallised from about 1 million years to over 4.5 billion years ago with routi ...
. Lead-207 exhibits
nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
, a property that has been used to study its compounds in solution and solid state, including in the human body.
Apart from the stable isotopes, which make up almost all lead that exists naturally, there are
trace quantities of a few radioactive isotopes. One of them is lead-210; although it has a half-life of only 22.2 years, small quantities occur in nature because lead-210 is produced by a long decay series that starts with uranium-238 (that has been present for billions of years on Earth). Lead-211, ŌłÆ212, and ŌłÆ214 are present in the decay chains of uranium-235, thorium-232, and uranium-238, respectively, so traces of all three of these lead isotopes are found naturally. Minute traces of lead-209 arise from the very rare
cluster decay
Cluster decay, also named heavy particle radioactivity, heavy ion radioactivity or heavy cluster decay," is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, ...
of radium-223, one of the
daughter products of natural uranium-235, the rare beta-minus-neutron decay of thallium-210 (a decay product of uranium-238), and the decay chain of neptunium-237, traces of which are produced by
neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
in uranium ores. Lead-213 also occurs in the decay chain of neptunium-237. Lead-210 is particularly useful for helping to identify the ages of samples by measuring its ratio to lead-206 (both isotopes are present in a single decay chain).
In total, 43 lead isotopes have been synthesized, with mass numbers 178ŌĆō220. Lead-205 is the most stable radioisotope, with a half-life of around 1.70 years. The second-most stable is lead-202, which has a half-life of about 52,500 years, longer than any of the natural trace radioisotopes.
Chemistry
Bulk lead exposed to moist air forms a protective layer of varying composition.
Lead(II) carbonate is a common constituent; the
sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
or
chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
may also be present in urban or maritime settings. This layer makes bulk lead effectively chemically inert in the air. Finely powdered lead, as with many metals, is
pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
, and burns with a bluish-white flame.
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
reacts with lead at room temperature, forming
lead(II) fluoride
Lead(II) fluoride is the inorganic compound with the formula Pb F2. It is a white solid. The compound is polymorphic, at ambient temperatures it exists in orthorhombic (PbCl2 type) form, while at high temperatures it is cubic ( Fluorite type). ...
. The reaction with
chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
is similar but requires heating, as the resulting chloride layer diminishes the reactivity of the elements. Molten lead reacts with the
chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
s to give lead(II) chalcogenides.
Lead metal resists
sulfuric
Sulfur (American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form ...
and
phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
but not
hydrochloric or
nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
; the outcome depends on insolubility and subsequent passivation of the product salt. Organic acids, such as
acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, dissolve lead in the presence of oxygen. Concentrated
alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
s dissolve lead and form
plumbite In chemistry, plumbite is the oxyanion or hydrated forms, or any salt containing this anion. In these salts, lead is in the oxidation state +2. It is the traditional term for the IUPAC name plumbate(II).
For example, lead(II) oxide (PbO) dissolves ...
s.
Inorganic compounds
Lead shows two main oxidation states: +4 and +2. The
tetravalent state is common for the carbon group. The divalent state is rare for
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalentŌĆömeaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
and
silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, minor for germanium, important (but not prevailing) for tin, and is the more important of the two oxidation states for lead. This is attributable to
relativistic effects, specifically the
inert pair effect
The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of o ...
, which manifests itself when there is a large difference in
electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
between lead and
oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of ŌłÆ2) of oxygen, an O2ŌłÆ ion with oxygen in the oxidation st ...
,
halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
, or
nitride
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3ŌłÆ, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitr ...
anions, leading to a significant partial positive charge on lead. The result is a stronger contraction of the lead 6s orbital than is the case for the 6p orbital, making it rather inert in ionic compounds. The inert pair effect is less applicable to compounds in which lead forms covalent bonds with elements of similar electronegativity, such as carbon in organolead compounds. In these, the 6s and 6p orbitals remain similarly sized and sp
3 hybridization is still energetically favorable. Lead, like carbon, is predominantly tetravalent in such compounds.
There is a relatively large difference in the electronegativity of lead(II) at 1.87 and lead(IV) at 2.33. This difference marks the reversal in the trend of increasing stability of the +4 oxidation state going down the carbon group; tin, by comparison, has values of 1.80 in the +2 oxidation state and 1.96 in the +4 state.
Lead(II)
Lead(II) compounds are characteristic of the inorganic chemistry of lead. Even strong
oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
s like fluorine and chlorine react with lead to give only
PbF2 and
PbCl2. Lead(II) ions are usually colorless in solution, and partially hydrolyze to form Pb(OH)
+ and finally
b4(OH)4sup>4+ (in which the
hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
ions act as
bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
s), but are not
reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
s as tin(II) ions are.
Techniques for identifying the presence of the Pb
2+ ion in water generally rely on the precipitation of lead(II) chloride using dilute hydrochloric acid. As the chloride salt is sparingly soluble in water, in very dilute solutions the precipitation of
lead(II) sulfide
Lead(II) sulfide (also spelled '' sulphide'') is an inorganic compound with the formula Pb S. Galena is the principal ore and the most important compound of lead. It is a semiconducting material with niche uses.
Formation, basic properties, rel ...
is instead achieved by bubbling
hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
through the solution.
Lead monoxide exists in two
polymorphs,
litharge
Litharge (from Greek , 'stone' + 'silver' ) is one of the natural mineral forms of lead(II) oxide, PbO. Litharge is a secondary mineral which forms from the oxidation of galena ores. It forms as coatings and encrustations with internal tetr ...
╬▒-PbO (red) and
massicot
Massicot is lead (II) oxide mineral with an orthorhombic lattice structure.
Lead(II) oxide (formula: PbO) can occur in one of two lattice formats, orthorhombic and tetragonal. The red tetragonal form is called litharge. PbO can be changed from m ...
╬▓-PbO (yellow), the latter being stable only above around 488 ┬░C. Litharge is the most commonly used inorganic compound of lead. There is no lead(II) hydroxide; increasing the pH of solutions of lead(II) salts leads to hydrolysis and condensation. Lead commonly reacts with heavier chalcogens.
Lead sulfide Lead sulfide refers to two compounds containing lead and sulfur:
*Lead(II) sulfide
Lead(II) sulfide (also spelled '' sulphide'') is an inorganic compound with the formula Pb S. Galena is the principal ore and the most important compound of lead ...
is a
semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
, a
photoconductor, and an extremely sensitive
infrared radiation detector. The other two chalcogenides,
lead selenide
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. (Note that incorrectly identifies PbSe and ...
and
lead telluride
Lead telluride is a compound of lead (element), lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.3 ...
, are likewise photoconducting. They are unusual in that their color becomes lighter going down the group.
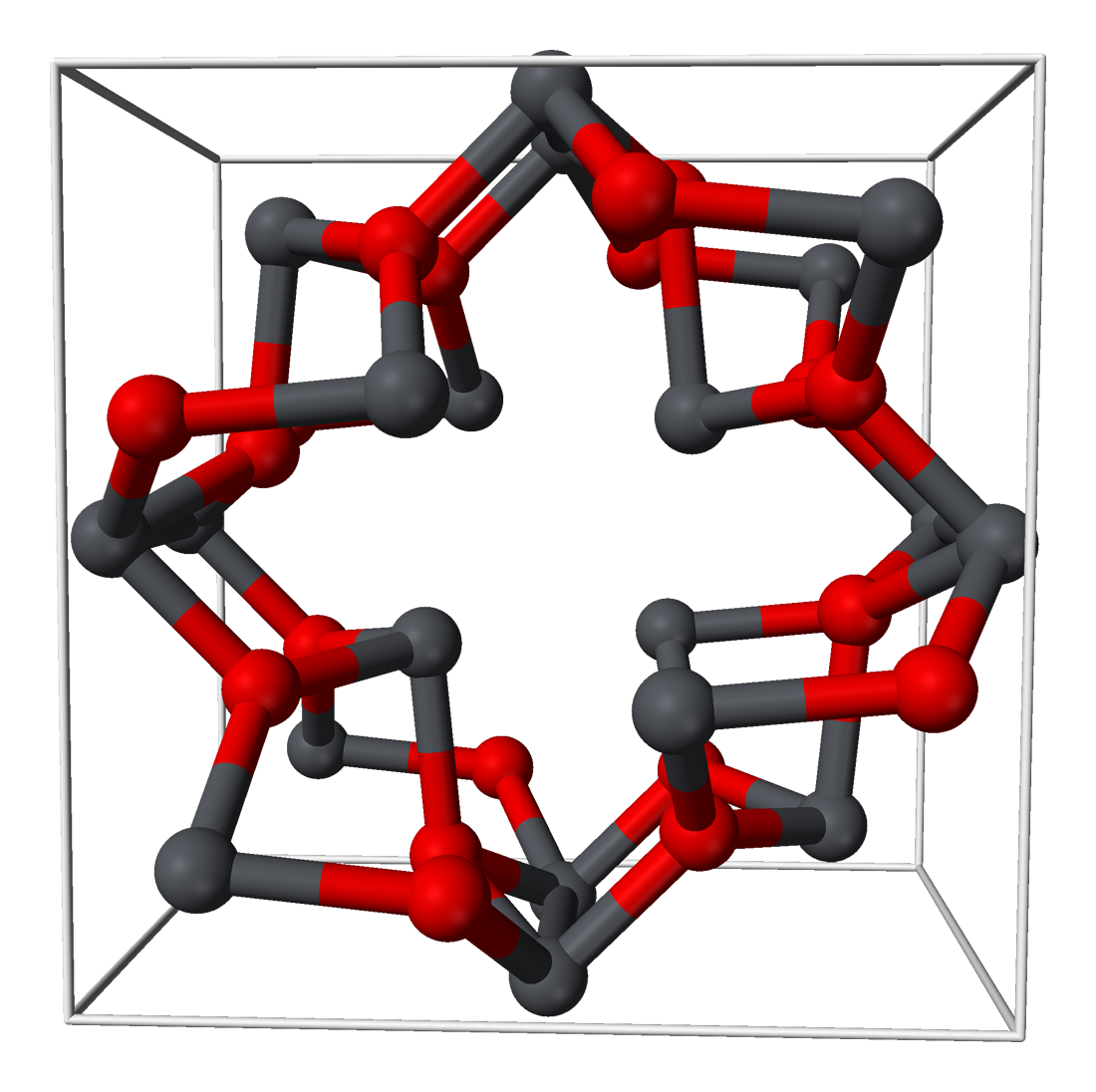
Lead dihalides are well-characterized; this includes the diastatide and mixed halides, such as PbFCl. The relative insolubility of the latter forms a useful basis for the
gravimetric determination of fluorine. The difluoride was the first solid
ionically conducting compound to be discovered (in 1834, by
Michael Faraday
Michael Faraday (; 22 September 1791 ŌĆō 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
). The other dihalides decompose on exposure to ultraviolet or visible light, especially
the diiodide. Many lead(II)
pseudohalides are known, such as the cyanide, cyanate, and
thiocyanate
Thiocyanates are salts containing the thiocyanate anion (also known as rhodanide or rhodanate). is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) t ...
. Lead(II) forms an extensive variety of halide
coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es, such as
bCl4sup>2ŌłÆ,
bCl6sup>4ŌłÆ, and the
b2Cl9sub>''n''
5''n''ŌłÆ chain anion.
Lead(II) sulfate
Lead(II) sulfate (PbSO4) is a white solid, which appears white in microcrystalline form. It is also known as ''fast white'', ''milk white'', ''sulfuric acid lead salt'' or ''anglesite''.
It is often seen in the plates/electrodes of car batteries ...
is insoluble in water, like the sulfates of other heavy divalent
cations
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
.
Lead(II) nitrate
Lead(II) nitrate is an inorganic compound with the chemical formula Pb( NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
Known since the Middle Ages by the name plumbum ...
and
lead(II) acetate
Lead(II) acetate is a white crystalline chemical compound with a slightly sweet taste. Its chemical formula is usually expressed as or , where Ac represents the acetyl group. Like many other lead compounds, it causes lead poisoning. Lead aceta ...
are very soluble, and this is exploited in the synthesis of other lead compounds.
Lead(IV)
Few inorganic lead(IV) compounds are known. They are only formed in highly oxidizing solutions and do not normally exist under standard conditions. Lead(II) oxide gives a mixed oxide on further oxidation, Pb
3O
4. It is described as
lead(II,IV) oxide, or structurally 2PbO┬ĘPbO
2, and is the best-known mixed valence lead compound.
Lead dioxide
Lead(IV) oxide, commonly known as lead dioxide, is an inorganic compound with the chemical formula . It is an oxide where lead is in an oxidation state of +4. It is a dark-brown solid which is insoluble in water. It exists in two crystalline forms ...
is a strong oxidizing agent, capable of oxidizing hydrochloric acid to chlorine gas. This is because the expected PbCl
4 that would be produced is unstable and spontaneously decomposes to PbCl
2 and Cl
2. Analogously to
lead monoxide, lead dioxide is capable of forming
plumbate
In chemistry, a plumbate often refers to compounds that can be viewed as derivatives of the hypothetical anion.
Examples Halides
Salts of , , , etc. are labeled as iodoplumbates. Lead perovskite semiconductors are often described as plumbates.
...
anions.
Lead disulfide and lead diselenide are only stable at high pressures.
Lead tetrafluoride, a yellow crystalline powder, is stable, but less so than the
difluoride.
Lead tetrachloride (a yellow oil) decomposes at room temperature, lead tetrabromide is less stable still, and the existence of lead tetraiodide is questionable.
Other oxidation states

Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained, as an intermediate between lead(II) and lead(IV), in larger organolead complexes; this oxidation state is not stable, as both the lead(III) ion and the larger complexes containing it are
radicals
Radical (from Latin: ', root) may refer to:
Politics and ideology Politics
*Classical radicalism, the Radical Movement that began in late 18th century Britain and spread to continental Europe and Latin America in the 19th century
*Radical politics ...
. The same applies for lead(I), which can be found in such radical species.
Numerous mixed lead(II,IV) oxides are known. When PbO
2 is heated in air, it becomes Pb
12O
19 at 293 ┬░C, Pb
12O
17 at 351 ┬░C, Pb
3O
4 at 374 ┬░C, and finally PbO at 605 ┬░C. A further
sesquioxide
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides.
Many sesquioxid ...
, Pb
2O
3, can be obtained at high pressure, along with several non-stoichiometric phases. Many of them show defective
fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scal ...
structures in which some oxygen atoms are replaced by vacancies: PbO can be considered as having such a structure, with every alternate layer of oxygen atoms absent.
Negative oxidation states can occur as
Zintl phases, as either free lead anions, as in Ba
2Pb, with lead formally being lead(ŌłÆIV), or in oxygen-sensitive ring-shaped or polyhedral cluster ions such as the
trigonal bipyramidal Pb
52ŌłÆ ion, where two lead atoms are lead(ŌłÆI) and three are lead(0). In such anions, each atom is at a polyhedral vertex and contributes two electrons to each covalent bond along an edge from their sp
3 hybrid orbitals, the other two being an external
lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
. They may be made in
liquid ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula . A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, ...
via the reduction of lead by
sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
.
Organolead

Lead can form
multiply-bonded chains, a property it shares with its lighter
homologs
Homologous chromosomes or homologs are a set of one maternal and one paternal chromosome that pair up with each other inside a cell during meiosis. Homologs have the same genes in the same loci, where they provide points along each chromosome th ...
in the carbon group. Its capacity to do so is much less because the PbŌĆōPb
bond energy
In chemistry, bond energy (''BE'') is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase b ...
is over three and a half times lower than that of the
CŌĆōC bond. With itself, lead can build metalŌĆōmetal bonds of an order up to three. With carbon, lead forms organolead compounds similar to, but generally less stable than, typical organic compounds (due to the PbŌĆōC bond being rather weak). This makes the
organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
of lead far less wide-ranging than that of tin. Lead predominantly forms organolead(IV) compounds, even when starting with inorganic lead(II) reactants; very few organolead(II) compounds are known. The most well-characterized exceptions are Pb
H(SiMe3)2sub>2 and
plumbocene.
The lead analog of the simplest
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbonŌĆōhydrogen or carbonŌĆōcarbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
,
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, is
plumbane
Plumbane is an inorganic chemical compound with the chemical formula PbH. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamicall ...
. Plumbane may be obtained in a reaction between metallic lead and atomic hydrogen. Two simple derivatives,
tetramethyllead
Tetramethyllead, also called tetra methyllead and lead tetramethyl, is a chemical compound used as an antiknock additive for gasoline. It is a methyl radical synthon. Its use in gasoline is being phased out for environmental considerations.
Th ...
and
tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
, are the best-known
organolead compounds. These compounds are relatively stable: tetraethyllead only starts to decompose if heated or if exposed to sunlight or ultraviolet light. With sodium metal, lead readily forms an equimolar alloy that reacts with
alkyl halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
to form
organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
compounds such as tetraethyllead. The oxidizing nature of many organolead compounds is usefully exploited:
lead tetraacetate is an important laboratory reagent for oxidation in organic synthesis. Tetraethyllead, once added to automotive gasoline, was produced in larger quantities than any other organometallic compound, and is still widely used in
fuel for small aircraft.
Other organolead compounds are less chemically stable. For many organic compounds, a lead analog does not exist.
Origin and occurrence
In space
Lead's per-particle abundance in the
Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
is 0.121
ppb (parts per billion). This figure is two and a half times higher than that of
platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, eight times more than
mercury, and seventeen times more than
gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
. The amount of lead in the
universe
The universe is all of space and time and their contents. It comprises all of existence, any fundamental interaction, physical process and physical constant, and therefore all forms of matter and energy, and the structures they form, from s ...
is slowly increasing as most heavier atoms (all of which are unstable) gradually decay to lead. The abundance of lead in the Solar System since its formation 4.5 billion years ago has increased by about 0.75%. The Solar System abundances table shows that lead, despite its relatively high atomic number, is more prevalent than most other elements with atomic numbers greater than 40.
Primordial leadŌĆöwhich comprises the isotopes lead-204, lead-206, lead-207, and lead-208ŌĆöwas mostly created as a result of repetitive neutron capture processes occurring in stars. The two main modes of capture are the
s- and
r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for nucleosynthesis, the creation of approximately half of the Atomic nucleus, atomic nuclei Heavy meta ...
es.
In the s-process (s is for "slow"), captures are separated by years or decades, allowing less stable nuclei to undergo
beta decay
In nuclear physics, beta decay (╬▓-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
. A stable thallium-203 nucleus can capture a neutron and become thallium-204; this undergoes beta decay to give stable lead-204; on capturing another neutron, it becomes lead-205, which has a half-life of around 17 million years. Further captures result in lead-206, lead-207, and lead-208. On capturing another neutron, lead-208 becomes lead-209, which quickly decays into bismuth-209. On capturing another neutron, bismuth-209 becomes bismuth-210, and this beta decays to polonium-210, which alpha decays to lead-206. The cycle hence ends at lead-206, lead-207, lead-208, and bismuth-209.

In the r-process (r is for "rapid"), captures happen faster than nuclei can decay. This occurs in environments with a high neutron density, such as a
supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
or the merger of two
neutron star
A neutron star is the gravitationally collapsed Stellar core, core of a massive supergiant star. It results from the supernova explosion of a stellar evolution#Massive star, massive starŌĆöcombined with gravitational collapseŌĆöthat compresses ...
s. The neutron flux involved may be on the order of 10
22 neutrons per square centimeter per second. The r-process does not form as much lead as the s-process. It tends to stop once neutron-rich nuclei reach 126 neutrons. At this point, the neutrons are arranged in complete shells in the atomic nucleus, and it becomes harder to energetically accommodate more of them. When the neutron flux subsides, these nuclei beta decay into stable isotopes of
osmium
Osmium () is a chemical element; it has Symbol (chemistry), symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a Abundance of elements in Earth's crust, trace element in a ...
,
iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
,
platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
.
On Earth
Lead is classified as a
chalcophile under the
Goldschmidt classification, meaning it is generally found combined with sulfur. It rarely occurs in its
native
Native may refer to:
People
* '' Jus sanguinis'', nationality by blood
* '' Jus soli'', nationality by location of birth
* Indigenous peoples, peoples with a set of specific rights based on their historical ties to a particular territory
** Nat ...
, metallic form. Many lead minerals are relatively light and, over the course of the Earth's history, have remained in the
crust instead of sinking deeper into the Earth's interior. This accounts for lead's relatively high
crustal abundance of 14 ppm; it is the 36th most
abundant element in the crust.
The main lead-bearing mineral is
galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crysta ...
(PbS), which is mostly found with zinc ores. Most other lead minerals are related to galena in some way;
boulangerite, Pb
5Sb
4S
11, is a mixed sulfide derived from galena;
anglesite
Anglesite is a lead sulfate mineral with the chemical formula PbSO4. It occurs as an oxidation product of primary lead sulfide ore, galena. Anglesite occurs as prismatic orthorhombic crystals and earthy masses, and is isomorphous with barite and ...
, PbSO
4, is a product of galena oxidation; and
cerussite
Cerussite (also known as lead carbonate or white lead ore) is a mineral consisting of lead carbonate with the chemical formula PbCO3, and is an important ore of lead. The name is from the Latin ''cerussa'', white lead. ''Cerussa nativa'' was ...
or white lead ore, PbCO
3, is a
decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
product of galena.
Arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
,
tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
,
antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
,
silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
,
gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
,
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
,
bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
are common impurities in lead minerals.

World lead resources exceed two billion tons. Significant deposits are located in Australia, China, Ireland, Mexico, Peru, Portugal, Russia, United States. Global reservesŌĆöresources that are economically feasible to extractŌĆötotaled 88 million tons in 2016, of which
Australia
Australia, officially the Commonwealth of Australia, is a country comprising mainland Australia, the mainland of the Australia (continent), Australian continent, the island of Tasmania and list of islands of Australia, numerous smaller isl ...
had 35 million, China 17 million, Russia 6.4 million.
Typical background concentrations of lead do not exceed 0.1 ╬╝g/m
3 in the atmosphere; 100 mg/kg in soil; 4 mg/kg in vegetation, 5 ╬╝g/L in fresh water and seawater.
Etymology
The modern English word ''lead'' is of Germanic origin; it comes from the
Middle English
Middle English (abbreviated to ME) is a form of the English language that was spoken after the Norman Conquest of 1066, until the late 15th century. The English language underwent distinct variations and developments following the Old English pe ...
and
Old English
Old English ( or , or ), or Anglo-Saxon, is the earliest recorded form of the English language, spoken in England and southern and eastern Scotland in the Early Middle Ages. It developed from the languages brought to Great Britain by Anglo-S ...
(with the
macron above the "e" signifying that the vowel sound of that letter is long). The Old English word is derived from the hypothetical reconstructed
Proto-Germanic
Proto-Germanic (abbreviated PGmc; also called Common Germanic) is the linguistic reconstruction, reconstructed proto-language of the Germanic languages, Germanic branch of the Indo-European languages.
Proto-Germanic eventually developed from ...
('lead'). According to linguistic theory, this word bore descendants in multiple Germanic languages of exactly the same meaning.
There is no consensus on the origin of the Proto-Germanic . One hypothesis suggests it is derived from
Proto-Indo-European
Proto-Indo-European (PIE) is the reconstructed common ancestor of the Indo-European language family. No direct record of Proto-Indo-European exists; its proposed features have been derived by linguistic reconstruction from documented Indo-Euro ...
('lead'; capitalization of the vowel is equivalent to the macron). Another hypothesis suggests it is borrowed from
Proto-Celtic
Proto-Celtic, or Common Celtic, is the hypothetical ancestral proto-language of all known Celtic languages, and a descendant of Proto-Indo-European. It is not attested in writing but has been partly Linguistic reconstruction, reconstructed throu ...
('lead'). This word is related to the
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
, which gave the element its
chemical symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist ...
''Pb''. The word is thought to be the origin of Proto-Germanic (which also means 'lead'), from which stemmed the German .
The name of the chemical element is not related to the verb of the same spelling, which is derived from Proto-Germanic ('to lead').
History
Prehistory and early history

Metallic lead beads
dating back to 7000ŌĆō6500 BC have been found in
Asia Minor
Anatolia (), also known as Asia Minor, is a peninsula in West Asia that makes up the majority of the land area of Turkey. It is the westernmost protrusion of Asia and is geographically bounded by the Mediterranean Sea to the south, the Aegean ...
and may represent the first example of metal
smelting
Smelting is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product. It is a form of extractive metallurgy that is used to obtain many metals such as iron-making, iron, copper extraction, copper ...
. At that time, lead had few (if any) applications due to its softness and dull appearance. The major reason for the spread of lead production was its association with silver, which may be obtained by burning galena (a common lead mineral). The
Ancient Egypt
Ancient Egypt () was a cradle of civilization concentrated along the lower reaches of the Nile River in Northeast Africa. It emerged from prehistoric Egypt around 3150BC (according to conventional Egyptian chronology), when Upper and Lower E ...
ians were the first to use lead minerals in cosmetics, an application that spread to
Ancient Greece
Ancient Greece () was a northeastern Mediterranean civilization, existing from the Greek Dark Ages of the 12thŌĆō9th centuries BC to the end of classical antiquity (), that comprised a loose collection of culturally and linguistically r ...
and beyond; the Egyptians had used lead for sinkers in
fishing nets
A fishing net or fish net is a net (device), net used for fishing. Fishing nets work by serving as an improvised fish trap, and some are indeed rigged as traps (e.g. #Fyke nets, fyke nets). They are usually wide open when deployed (e.g. by cast ...
,
glazes,
glasses
Glasses, also known as eyeglasses (American English), spectacles (Commonwealth English), or colloquially as specs, are vision eyewear with clear or tinted lenses mounted in a frame that holds them in front of a person's eyes, typically u ...
,
enamels,
ornaments
An ornament is something used for decoration.
Ornament may also refer to:
Decoration
*Ornament (art), any purely decorative element in architecture and the decorative arts
*Ornamental turning
*Biological ornament, a characteristic of animals tha ...
. Various civilizations of the
Fertile Crescent
The Fertile Crescent () is a crescent-shaped region in the Middle East, spanning modern-day Iraq, Israel, Jordan, Lebanon, Palestine, and Syria, together with northern Kuwait, south-eastern Turkey, and western Iran. Some authors also include ...
used lead as a
writing material
A writing material, also called a writing medium, is a surface that can be written on with suitable instruments, or used for symbolic or representational drawings. Building materials on which writings or drawings are produced are not included. ...
, as
coin
A coin is a small object, usually round and flat, used primarily as a medium of exchange or legal tender. They are standardized in weight, and produced in large quantities at a mint in order to facilitate trade. They are most often issued by ...
s, and as a
construction material
This is a list of building materials.
Many types of building materials are used in the construction industry to create buildings and structures. These categories of materials and products are used by architects and construction project manager
...
. Lead was used by the ancient Chinese as a
stimulant
Stimulants (also known as central nervous system stimulants, or psychostimulants, or colloquially as uppers) are a class of drugs that increase alertness. They are used for various purposes, such as enhancing attention, motivation, cognition, ...
, as
currency
A currency is a standardization of money in any form, in use or circulation as a medium of exchange, for example banknotes and coins. A more general definition is that a currency is a ''system of money'' in common use within a specific envi ...
, as
contraceptive
Birth control, also known as contraception, anticonception, and fertility control, is the use of methods or devices to prevent pregnancy. Birth control has been used since ancient times, but effective and safe methods of birth control only be ...
, and in
chopsticks
Chopsticks are shaped pairs of equal-length sticks that have been used as kitchen and eating utensils in most of East Asia for over three millennia. They are held in the dominant hand, secured by fingers, and wielded as extensions of the han ...
. The
Indus Valley civilization
The Indus Valley Civilisation (IVC), also known as the Indus Civilisation, was a Bronze Age civilisation in the northwestern regions of South Asia, lasting from 3300 BCE to 1300 BCE, and in its mature form from 2600 BCE ...
and the
Mesoamerica
Mesoamerica is a historical region and cultural area that begins in the southern part of North America and extends to the Pacific coast of Central America, thus comprising the lands of central and southern Mexico, all of Belize, Guatemala, El S ...
ns used it for making
amulets
An amulet, also known as a good luck charm or phylactery, is an object believed to confer protection upon its possessor. The word "amulet" comes from the Latin word , which Pliny's ''Natural History'' describes as "an object that protects a pers ...
; and the eastern and southern Africans used lead in
wire drawing
Wire drawing is a metalworking process used to reduce the cross-section of a wire by pulling the wire through one or more dies. There are many applications for wire drawing, including electrical wiring, cables, tension-loaded structural compone ...
.
Classical era
Because silver was extensively used as a decorative material and an exchange medium, lead deposits came to be worked in Asia Minor from 3000 BC; later, lead deposits were developed in the
Aegean and
Laurion
Lavrio, Lavrion or Laurium (; (later ); from Middle Ages until 1908: ╬ĢŽü╬│╬▒ŽāŽä╬«Žü╬╣╬▒ ''Ergastiria'') is a town in southeastern part of Attica Region, Attica, Greece. It is part of Athens metropolitan area and the seat of the municipalit ...
. These three regions collectively dominated production of mined lead until . Beginning c. 2000 BC, the
Phoenicia
Phoenicians were an Ancient Semitic-speaking peoples, ancient Semitic group of people who lived in the Phoenician city-states along a coastal strip in the Levant region of the eastern Mediterranean, primarily modern Lebanon and the Syria, Syrian ...
ns worked deposits in the
Iberian peninsula
The Iberian Peninsula ( ), also known as Iberia, is a peninsula in south-western Europe. Mostly separated from the rest of the European landmass by the Pyrenees, it includes the territories of peninsular Spain and Continental Portugal, comprisin ...
; by 1600 BC, lead mining existed in
Cyprus
Cyprus (), officially the Republic of Cyprus, is an island country in the eastern Mediterranean Sea. Situated in West Asia, its cultural identity and geopolitical orientation are overwhelmingly Southeast European. Cyprus is the List of isl ...
,
Greece
Greece, officially the Hellenic Republic, is a country in Southeast Europe. Located on the southern tip of the Balkan peninsula, it shares land borders with Albania to the northwest, North Macedonia and Bulgaria to the north, and Turkey to th ...
, and
Sardinia
Sardinia ( ; ; ) is the Mediterranean islands#By area, second-largest island in the Mediterranean Sea, after Sicily, and one of the Regions of Italy, twenty regions of Italy. It is located west of the Italian Peninsula, north of Tunisia an ...
.
 Rome's
Rome's territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the
classical era
Classical antiquity, also known as the classical era, classical period, classical age, or simply antiquity, is the period of cultural European history between the 8th century BC and the 5th century AD comprising the interwoven civilization ...
, with an estimated annual output peaking at 80,000 tonnes. Like their predecessors, the Romans obtained lead mostly as a by-product of silver smelting. Lead mining occurred in
central Europe
Central Europe is a geographical region of Europe between Eastern Europe, Eastern, Southern Europe, Southern, Western Europe, Western and Northern Europe, Northern Europe. Central Europe is known for its cultural diversity; however, countries in ...
,
Britain
Britain most often refers to:
* Great Britain, a large island comprising the countries of England, Scotland and Wales
* The United Kingdom of Great Britain and Northern Ireland, a sovereign state in Europe comprising Great Britain and the north-eas ...
,
Balkans
The Balkans ( , ), corresponding partially with the Balkan Peninsula, is a geographical area in southeastern Europe with various geographical and historical definitions. The region takes its name from the Balkan Mountains that stretch throug ...
,
Greece
Greece, officially the Hellenic Republic, is a country in Southeast Europe. Located on the southern tip of the Balkan peninsula, it shares land borders with Albania to the northwest, North Macedonia and Bulgaria to the north, and Turkey to th ...
,
Anatolia
Anatolia (), also known as Asia Minor, is a peninsula in West Asia that makes up the majority of the land area of Turkey. It is the westernmost protrusion of Asia and is geographically bounded by the Mediterranean Sea to the south, the Aegean ...
,
Hispania
Hispania was the Ancient Rome, Roman name for the Iberian Peninsula. Under the Roman Republic, Hispania was divided into two Roman province, provinces: Hispania Citerior and Hispania Ulterior. During the Principate, Hispania Ulterior was divide ...
, the latter accounting for 40% of world production.

Lead tablets were commonly used as a material for letters. Lead coffins, cast in flat sand forms and with interchangeable motifs to suit the faith of the deceased, were used in ancient
Judea
Judea or Judaea (; ; , ; ) is a mountainous region of the Levant. Traditionally dominated by the city of Jerusalem, it is now part of Palestine and Israel. The name's usage is historic, having been used in antiquity and still into the pres ...
. Lead was used to make sling bullets from the 5th century BC. In Roman times, lead sling bullets were amply used, and were effective at a distance of between 100 and 150 meters. The
Balearic slingers Balearic may refer to:
*Of the Balearic Islands
*The Balearic dialect of Catalan
*Balearic horse, a term sometimes used to describe either or both of these horse breeds in the region:
** Mallorqu├Łn
** Menorqu├Łn horse
*Balearic beat
Balearic b ...
, used as mercenaries in Carthaginian and Roman armies, were famous for their shooting distance and accuracy.
Lead was used for making water pipes in the
Roman Empire
The Roman Empire ruled the Mediterranean and much of Europe, Western Asia and North Africa. The Roman people, Romans conquered most of this during the Roman Republic, Republic, and it was ruled by emperors following Octavian's assumption of ...
; the
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
word for the metal, , is the origin of the English word "
plumbing
Plumbing is any system that conveys fluids for a wide range of applications. Plumbing uses piping, pipes, valves, piping and plumbing fitting, plumbing fixtures, Storage tank, tanks, and other apparatuses to convey fluids. HVAC, Heating and co ...
". Its ease of working, its low melting point enabling the easy fabrication of completely waterproof welded joints, and its resistance to corrosion ensured its widespread use in other applications, including pharmaceuticals, roofing, currency, warfare. Writers of the time, such as
Cato the Elder
Marcus Porcius Cato (, ; 234ŌĆō149 BC), also known as Cato the Censor (), the Elder and the Wise, was a Roman soldier, Roman Senate, senator, and Roman historiography, historian known for his conservatism and opposition to Hellenization. He wa ...
,
Columella
Lucius Junius Moderatus Columella (, Arabic: ) was a prominent Roman writer on agriculture in the Roman Empire.
His in twelve volumes has been completely preserved and forms an important source on Roman agriculture and ancient Roman cuisin ...
, and
Pliny the Elder
Gaius Plinius Secundus (AD 23/24 79), known in English as Pliny the Elder ( ), was a Roman Empire, Roman author, Natural history, naturalist, and naval and army commander of the early Roman Empire, and a friend of the Roman emperor, emperor Vesp ...
, recommended lead (and lead-coated) vessels for the preparation of
sweeteners and preservatives added to wine and food. The lead conferred an agreeable taste due to the formation of "sugar of lead" (
lead(II) acetate
Lead(II) acetate is a white crystalline chemical compound with a slightly sweet taste. Its chemical formula is usually expressed as or , where Ac represents the acetyl group. Like many other lead compounds, it causes lead poisoning. Lead aceta ...
), whereas
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
vessels imparted a bitter flavor through
verdigris
Verdigris () is a common name for any of a variety of somewhat toxic copper salt (chemistry), salts of acetic acid, which range in colour from green to a blue-green, bluish-green depending on their chemical composition.H. K├╝hn, Verdigris and Cop ...
formation.
The Roman author
Vitruvius
Vitruvius ( ; ; ŌĆō70 BC ŌĆō after ) was a Roman architect and engineer during the 1st century BC, known for his multi-volume work titled . As the only treatise on architecture to survive from antiquity, it has been regarded since the Renaissan ...
reported the
health dangers of lead and modern writers have suggested that lead poisoning played a major role in the
decline of the Roman Empire
The fall of the Western Roman Empire, also called the fall of the Roman Empire or the fall of Rome, was the loss of central political control in the Western Roman Empire, a process in which the Empire failed to enforce its rule, and its vast ...
. Other researchers have criticized such claims, pointing out, for instance, that not all
abdominal pain
Abdominal pain, also known as a stomach ache, is a symptom associated with both non-serious and serious medical issues. Since the abdomen contains most of the body's vital organs, it can be an indicator of a wide variety of diseases. Given th ...
is caused by lead poisoning. According to archaeological research, Roman
lead pipes increased lead levels in tap water but such an effect was "unlikely to have been truly harmful". When lead poisoning did occur, victims were called "saturnine", dark and cynical, after the ghoulish father of the gods,
Saturn
Saturn is the sixth planet from the Sun and the second largest in the Solar System, after Jupiter. It is a gas giant, with an average radius of about 9 times that of Earth. It has an eighth the average density of Earth, but is over 95 tim ...
. By association, lead was considered the father of all metals. Its status in Roman society was low as it was readily available and cheap.
Confusion with tin and antimony
Since the
Bronze Age
The Bronze Age () was a historical period characterised principally by the use of bronze tools and the development of complex urban societies, as well as the adoption of writing in some areas. The Bronze Age is the middle principal period of ...
, metallurgists and engineers have understood the difference between rare and valuable
tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
, essential for alloying with copper to produce tough and corrosion resistant
bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12ŌĆō12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals (such as phosphorus) or metalloid ...
, and 'cheap and cheerful' lead. However, the nomenclature in some languages is similar. Romans called lead ("black lead"), and tin ("bright lead"). The association of lead and tin can be seen in other languages: the word in
Czech
Czech may refer to:
* Anything from or related to the Czech Republic, a country in Europe
** Czech language
** Czechs, the people of the area
** Czech culture
** Czech cuisine
* One of three mythical brothers, Lech, Czech, and Rus
*Czech (surnam ...
translates to "lead", but in Russian, its
cognate
In historical linguistics, cognates or lexical cognates are sets of words that have been inherited in direct descent from an etymological ancestor in a common parent language.
Because language change can have radical effects on both the s ...
() means "tin". To add to the confusion, lead bore a close relation to antimony: both elements commonly occur as sulfides (galena and
stibnite
Stibnite, sometimes called antimonite, is a sulfide mineral, a mineral form of antimony trisulfide ( Sb2 S3). It is a soft, metallic grey crystalline solid with an orthorhombic space group. It is the most important source for the metalloid an ...
), often together. Pliny incorrectly wrote that stibnite would give lead on heating, instead of antimony. In countries such as Turkey and India, the originally Persian name came to refer to either antimony sulfide or lead sulfide, and in some languages, such as Russian, gave its name to antimony ().
Middle Ages and the Renaissance
Lead mining in Western Europe declined after the fall of the
Western Roman Empire
In modern historiography, the Western Roman Empire was the western provinces of the Roman Empire, collectively, during any period in which they were administered separately from the eastern provinces by a separate, independent imperial court. ...
, with
Arabian Iberia being the only region having a significant output. The largest production of lead occurred in South Asia and East Asia, especially China and India, where lead mining grew rapidly.
In Europe, lead production began to increase in the 11th and 12th centuries, when it was again used for roofing and piping. Starting in the 13th century, lead was used to create
stained glass
Stained glass refers to coloured glass as a material or art and architectural works created from it. Although it is traditionally made in flat panels and used as windows, the creations of modern stained glass artists also include three-dimensio ...
. In the
European and
Arabian
The Arabian Peninsula (, , or , , ) or Arabia, is a peninsula in West Asia, situated north-east of Africa on the Arabian plate. At , comparable in size to India, the Arabian Peninsula is the largest peninsula in the world.
Geographically, the ...
traditions of
alchemy
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
, lead (symbol ŌÖä in the European tradition) was considered an impure
base metal
A base metal is a common and inexpensive metal, as opposed to a precious metal such as gold or silver. In numismatics, coins often derived their value from the precious metal content; however, base metals have also been used in coins in the past ...
which, by the separation, purification and balancing of its constituent essences, could be transformed to pure and incorruptible gold. During the period, lead was used increasingly for
adulterating wine. The use of such wine was forbidden for use in Christian rites by a
papal bull
A papal bull is a type of public decree, letters patent, or charter issued by the pope of the Catholic Church. It is named after the leaden Seal (emblem), seal (''bulla (seal), bulla'') traditionally appended to authenticate it.
History
Papal ...
in 1498, but it continued to be imbibed and resulted in mass poisonings up to the late 18th century. Lead was a key material in parts of the
printing press
A printing press is a mechanical device for applying pressure to an inked surface resting upon a printing, print medium (such as paper or cloth), thereby transferring the ink. It marked a dramatic improvement on earlier printing methods in whi ...
, and lead dust was commonly inhaled by print workers, causing lead poisoning. Lead also became the chief material for making bullets for firearms: it was cheap, less damaging to iron gun barrels, had a higher density (which allowed for better retention of velocity), and its lower melting point made the production of bullets easier as they could be made using a wood fire. Lead, in the form of
Venetian ceruse, was extensively used in cosmetics by Western European aristocracy as whitened faces were regarded as a sign of modesty. This practice later expanded to white wigs and eyeliners, and only faded out with the
French Revolution in the late 18th century. A similar fashion appeared in Japan in the 18th century with the emergence of the
geisha
{{Culture of Japan, Traditions, Geisha
{{nihongo, Geisha{{efn, {{IPAc-en, lang, ╦ł, ╔Ī, e╔¬, ., ╩ā, ╔Ö, {{IPA, ja, ╔Īei.╔Ģa, ╔Īe╦É-, lang{{cite book, script-title=ja:NHKµŚźµ£¼Ķ¬×ńÖ║ķ¤│ŃéóŃé»Ńé╗Ńā│Ńāłµ¢░ĶŠ×ÕģĖ, publisher=NHK Publishing, editor= ...
s, a practice that continued long into the 20th century. The white faces of women "came to represent their feminine virtue as Japanese women", with lead commonly used in the whitener.
Outside Europe and Asia
In the
New World
The term "New World" is used to describe the majority of lands of Earth's Western Hemisphere, particularly the Americas, and sometimes Oceania."America." ''The Oxford Companion to the English Language'' (). McArthur, Tom, ed., 1992. New York: ...
, lead production was recorded soon after the arrival of European settlers. The earliest record dates to 1621 in the English
Colony of Virginia
The Colony of Virginia was a British Empire, British colonial settlement in North America from 1606 to 1776.
The first effort to create an English settlement in the area was chartered in 1584 and established in 1585; the resulting Roanoke Colo ...
, fourteen years after its foundation. In Australia, the first mine opened by colonists on the continent was a lead mine, in 1841. In Africa, lead mining and smelting were known in the
Benue Trough
The Benue Trough is a major geological structure underlying a large part of Nigeria and extending about 1,000 km northeast from the Bight of Benin to Lake Chad. It is part of the broader West and Central African Rift System.
Location
The ...
and the lower
Congo Basin
The Congo Basin () is the sedimentary basin of the Congo River. The Congo Basin is located in Central Africa, in a region known as west equatorial Africa. The Congo Basin region is sometimes known simply as the Congo. It contains some of the larg ...
, where lead was used for trade with Europeans, and as a currency by the 17th century, well before the
scramble for Africa
The Scramble for Africa was the invasion, conquest, and colonialism, colonisation of most of Africa by seven Western European powers driven by the Second Industrial Revolution during the late 19th century and early 20th century in the era of ...
.
Industrial Revolution

In the second half of the 18th century, Britain, and later continental Europe and the United States, experienced the
Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
. This was the first time during which lead production rates exceeded those of Rome. Britain was the leading producer, losing this status by the mid-19th century with the depletion of its mines and the development of lead mining in Germany, Spain, and the United States. By 1900, the United States was the leader in global lead production, and other non-European nationsŌĆöCanada, Mexico, and AustraliaŌĆöhad begun significant production; production outside Europe exceeded that within. A great share of the demand for lead came from plumbing and paintingŌĆö
lead paint
Lead paint or lead-based paint is paint containing lead. As pigment, lead(II) chromate (, "chrome yellow"), lead(II,IV) oxide, (, "red lead"), and lead(II) carbonate (, "white lead") are the most common forms.. Lead is added to paint to acceler ...
s were in regular use. At this time, more (working class) people were exposed to the metal and lead poisoning cases escalated. This led to research into the effects of lead intake. Lead was proven to be more dangerous in its fume form than as a solid metal. Lead poisoning and
gout
Gout ( ) is a form of inflammatory arthritis characterized by recurrent attacks of pain in a red, tender, hot, and Joint effusion, swollen joint, caused by the deposition of needle-like crystals of uric acid known as monosodium urate crysta ...
were linked; British physician
Alfred Baring Garrod noted a third of his gout patients were plumbers and painters. The effects of chronic ingestion of lead, including mental disorders, were also studied in the 19th century. The first laws aimed at decreasing lead poisoning in factories were enacted during the 1870s and 1880s in the United Kingdom.
Modern era

Further evidence of the threat that lead posed to humans was discovered in the late 19th and early 20th centuries. Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe. The United Kingdom introduced mandatory factory inspections in 1878 and appointed the first Medical Inspector of Factories in 1898; as a result, a 25-fold decrease in lead poisoning incidents from 1900 to 1944 was reported. Most European countries banned lead paintŌĆöcommonly used because of its opacity and water resistanceŌĆöfor interiors by 1930.
The last major human exposure to lead was the addition of
tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
to gasoline as an
antiknock agent
An antiknock agent (also: knock inhibitor) is a gasoline additive used to reduce engine knocking and increase the fuel's octane rating by raising the temperature and pressure at which auto-ignition occurs. The mixture known as gasoline or petrol, ...
, a practice that originated in the United States in 1921. It was phased out in the United States and the
European Union
The European Union (EU) is a supranational union, supranational political union, political and economic union of Member state of the European Union, member states that are Geography of the European Union, located primarily in Europe. The u ...
by 2000.
In the 1970s, the United States and Western European countries introduced legislation to reduce lead air pollution. The impact was significant: while a study conducted by the
Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention (CDC) is the National public health institutes, national public health agency of the United States. It is a Federal agencies of the United States, United States federal agency under the United S ...
in the United States in 1976ŌĆō1980 showed that 77.8% of the population had elevated
blood lead level
Blood lead level (BLL), is a measure of the amount of lead in the blood. Lead is a toxic heavy metal and can cause neurological damage, especially among children, at any detectable level. High lead levels cause decreased vitamin D and haemo ...
s, in 1991ŌĆō1994, a study by the same institute showed the share of people with such high levels dropped to 2.2%. The main product made of lead by the end of the 20th century was the
leadŌĆōacid battery
The leadŌĆōacid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Plant├®. It was the first type of rechargeable battery to be invented. Compared to modern rechargeable batteries, leadŌĆōacid batteries ha ...
.
From 1960 to 1990, lead output in the
Western Bloc
The Western Bloc, also known as the Capitalist Bloc, the Freedom Bloc, the Free Bloc, and the American Bloc, was an unofficial coalition of countries that were officially allied with the United States during the Cold War (1947ŌĆō1991). While ...
grew by about 31%. The share of the world's lead production by the
Eastern Bloc
The Eastern Bloc, also known as the Communist Bloc (Combloc), the Socialist Bloc, the Workers Bloc, and the Soviet Bloc, was an unofficial coalition of communist states of Central and Eastern Europe, Asia, Africa, and Latin America that were a ...
increased from 10% to 30%, from 1950 to 1990, with the
Soviet Union
The Union of Soviet Socialist Republics. (USSR), commonly known as the Soviet Union, was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 until Dissolution of the Soviet ...
being the world's largest producer during the mid-1970s and the 1980s, and China starting major lead production in the late 20th century. Unlike the European communist countries, China was largely unindustrialized by the mid-20th century; in 2004, China surpassed Australia as the largest producer of lead. As was the case during European industrialization, lead has had a negative effect on health in China.
Production

As of 2014, production of lead is increasing worldwide due to its use in leadŌĆōacid batteries. There are two major categories of production: primary from mined ores, and secondary from scrap. In 2014, 4.58 million metric tons came from primary production and 5.64 million from secondary production. The top three producers of mined lead concentrate in that year were China, Australia, and United States. The top three producers of refined lead were China, United States, and India. According to the
Metal Stocks in Society report of 2010, the total amount of lead in use, stockpiled, discarded, or dissipated into the environment, on a global basis, is 8 kg per capita. Much of this is in more developed countries (20ŌĆō150 kg per capita) rather than less developed ones (1ŌĆō4 kg per capita).
The primary and secondary lead production processes are similar. Some primary production plants now supplement their operations with scrap lead, and this trend is likely to increase in the future. Given adequate techniques, lead obtained via secondary processes is indistinguishable from lead obtained via primary processes. Scrap lead from the building trade is usually fairly clean and is re-melted without the need for smelting, though refining is sometimes needed. Secondary lead production is therefore cheaper, in terms of energy requirements, than is primary production, often by 50% or more.
Primary
Most lead ores contain a low percentage of lead (rich ores have a typical content of 3ŌĆō8%) which must be concentrated for extraction. During initial processing, ores typically undergo crushing, dense-medium separation,
grinding,
froth flotation,
drying
Drying is a mass transfer process consisting of the removal of water or another solvent by evaporation from a solid, semi-solid or liquid. This process is often used as a final production step before selling or packaging products. To be consider ...
. The resulting concentrate, which has a lead content of 30ŌĆō80% by mass (regularly 50ŌĆō60%), is then turned into (impure) lead metal.
There are two main ways of doing this: a two-stage process involving roasting followed by blast furnace extraction, carried out in separate vessels; or a direct process in which the extraction of the concentrate occurs in a single vessel. The latter has become the most common route, though the former is still significant.
roasted
Roasting is a cooking method that uses dry heat where hot air covers the food, cooking it evenly on all sides with temperatures of at least from an open flame, oven, or other heat source. Roasting can enhance the flavor through caramelizat ...
in air to oxidize the lead sulfide:
: 2 PbS(s) + 3 O
2(g) ŌåÆ 2 PbO(s) + 2 SO
2(g)Ōåæ
As the original concentrate was not pure lead sulfide, roasting yields not only the desired lead(II) oxide, but a mixture of oxides, sulfates, and silicates of lead and of the other metals contained in the ore. This impure lead oxide is reduced in a
coke-fired blast furnace to the (again, impure) metal:
: 2 PbO(s) + C(s) ŌåÆ 2 Pb(s) + CO
2(g)Ōåæ
Impurities are mostly arsenic, antimony, bismuth, zinc, copper, silver, and gold. Typically they are removed in a series of
pyrometallurgical processes. The melt is treated in a
reverberatory furnace
A reverberatory furnace is a metallurgy, metallurgical or process Metallurgical furnace, furnace that isolates the material being processed from contact with the fuel, but not from contact with combustion gases. The term ''reverberation'' is use ...
with air, steam, sulfur, which oxidizes the impurities except for silver, gold, bismuth. Oxidized contaminants float to the
top of the melt and are skimmed off. Metallic silver and gold are removed and recovered economically by means of the
Parkes process
The Parkes process is a pyrometallurgical industrial process for removing silver from lead during the production of bullion. It is an example of liquidŌĆōliquid extraction.
The process takes advantage of two liquid-state properties of zinc. The fi ...
, in which zinc is added to lead. Zinc, which is immiscible in lead, dissolves the silver and gold. The zinc solution can be separated from the lead, and the silver and gold retrieved. De-silvered lead is freed of bismuth by the
BettertonŌĆōKroll process, treating it with metallic
calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
and
magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
. The resulting bismuth dross can be skimmed off.
Alternatively to the pyrometallurgical processes, very pure lead can be obtained by processing smelted lead electrolytically using the
Betts process. Anodes of impure lead and cathodes of pure lead are placed in an electrolyte of lead
fluorosilicate (PbSiF
6). Once electrical potential is applied, impure lead at the anode dissolves and plates onto the cathode, leaving the majority of the impurities in solution. This is a high-cost process and thus mostly reserved for refining bullion containing high percentages of impurities.
Direct process
In this process, lead bullion and
slag
The general term slag may be a by-product or co-product of smelting (pyrometallurgical) ores and recycled metals depending on the type of material being produced. Slag is mainly a mixture of metal oxides and silicon dioxide. Broadly, it can be c ...
is obtained directly from lead concentrates. The lead sulfide concentrate is melted in a furnace and oxidized, forming lead monoxide. Carbon (as coke or
coal gas
Coal gas is a flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. It is produced when coal is heated strongly in the absence of air. Town gas is a more general term referring to manufactured gaseous ...
) is added to the molten charge along with
fluxing agents. The lead monoxide is thereby reduced to metallic lead, in the midst of a slag rich in lead monoxide.
If the input is rich in lead, as much as 80% of the original lead can be obtained as bullion; the remaining 20% forms a slag rich in lead monoxide. For a low-grade feed, all of the lead can be oxidized to a high-lead slag. Metallic lead is further obtained from the high-lead (25ŌĆō40%) slags via submerged fuel combustion or injection, reduction assisted by an electric furnace, or a combination of both.
Alternatives
Research on a cleaner, less energy-intensive lead extraction process continues; a major drawback is that either too much lead is lost as waste, or the alternatives result in a high sulfur content in the resulting lead metal.
Hydrometallurgical extraction, in which
anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
s of impure lead are immersed into an
electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
and pure lead is deposited (
electrowound) onto
cathodes
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current ...
, is a technique that may have potential, but is not currently economical except in cases where electricity is very cheap.
Secondary
Smelting, which is an essential part of the primary production, is often skipped during secondary production. It is only performed when metallic lead has undergone significant oxidation. The process is similar to that of primary production in either a
blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being supplied above atmospheric pressure.
In a ...
or a
rotary furnace, with the essential difference being the greater variability of yields: blast furnaces produce hard lead (10% antimony) while reverberatory and rotary kiln furnaces produce semisoft lead (3ŌĆō4% antimony).
The
ISASMELT process is a more recent smelting method that may act as an extension to primary production; battery paste from spent leadŌĆōacid batteries (containing lead sulfate and lead oxides) has its sulfate removed by treating it with alkali, and is then treated in a coal-fueled furnace in the presence of oxygen, which yields impure lead, with antimony the most common impurity. Refining of secondary lead is similar to that of primary lead; some refining processes may be skipped depending on the material recycled and its potential contamination.
Of the sources of lead for recycling, leadŌĆōacid batteries are the most important; lead pipe, sheet, and cable sheathing are also significant.
Applications

Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. When the pencil originated as a wrapped graphite writing tool, the particular type of
graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
used was named ''
plumbago
''Plumbago'' is a genus of 23 species of flowering plants in the family Plumbaginaceae, native to warm temperate to tropical regions of the world. Common names include plumbago and leadwort (names which are also shared by the genus '' Ceratostig ...
'' (literally, ''lead mockup'').
Elemental form
Lead metal has several useful mechanical properties, including high density, low melting point, ductility, and relative inertness. Many metals are superior to lead in some of these aspects but are generally less common and more difficult to extract from parent ores. Lead's toxicity has led to its phasing out for some uses.
Lead has been used for bullets since their invention in the Middle Ages. It is inexpensive; its low melting point means small arms ammunition and shotgun pellets can be cast with minimal technical equipment; and it is denser than other common metals, which allows for better retention of velocity. It remains the main material for bullets, alloyed with other metals as hardeners. Concerns have been raised that lead bullets used for hunting can damage the environment.
Shotgun
A shotgun (also known as a scattergun, peppergun, or historically as a fowling piece) is a long gun, long-barreled firearm designed to shoot a straight-walled cartridge (firearms), cartridge known as a shotshell, which discharges numerous small ...
cartridges used for
waterfowl hunting
Waterfowl hunting is the practice of hunting aquatic birds such as ducks, geese and other waterfowls or shorebirds for sport and meat. Waterfowl are hunted in crop fields where they feed, or in areas with bodies of water such as rivers, lakes ...
must today be lead-free in the
United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
,
Canada
Canada is a country in North America. Its Provinces and territories of Canada, ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, making it the world's List of coun ...
, and in
Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east ...
.
Lead's high density and resistance to corrosion have been exploited in a number of related applications. It is used as
ballast
Ballast is dense material used as a weight to provide stability to a vehicle or structure. Ballast, other than cargo, may be placed in a vehicle, often a ship or the gondola of a balloon or airship, to provide stability. A compartment within ...
in sailboat keels; its density allows it to take up a small volume and minimize water resistance, thus counterbalancing the heeling effect of wind on the sails. It is used in
scuba diving
Scuba diving is a Diving mode, mode of underwater diving whereby divers use Scuba set, breathing equipment that is completely independent of a surface breathing gas supply, and therefore has a limited but variable endurance. The word ''scub ...
weight belts to counteract the diver's buoyancy. In 1993, the base of the
Leaning Tower of Pisa
The Leaning Tower of Pisa ( ), or simply the Tower of Pisa (), is the , or freestanding bell tower, of Pisa Cathedral. It is known for its nearly four-degree lean, the result of an unstable Foundation (engineering), foundation. The tower is on ...
was stabilized with 600 tonnes of lead. Because of its corrosion resistance, lead is used as a protective sheath for underwater cables.
Lead has many uses in the construction industry; lead sheets are used as
architectural metals in
roofing material
Domestic roof construction is the framing (construction), framing and roof covering which is found on most detached houses in cold and temperate climates. Such roofs are built with mostly timber, take a number of different List of roof shapes, ...
,
cladding,
flashing,
gutters and gutter joints, roof parapets. Lead is still used in statues and sculptures, including for
armatures. In the past it was often used to
balance the wheels of cars; for environmental reasons this use is being phased out in favor of other materials.
Lead is added to copper alloys, such as
brass
Brass is an alloy of copper and zinc, in proportions which can be varied to achieve different colours and mechanical, electrical, acoustic and chemical properties, but copper typically has the larger proportion, generally copper and zinc. I ...
and
bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12ŌĆō12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals (such as phosphorus) or metalloid ...
, to improve machinability and for its lubricating qualities. Being practically insoluble in copper, the lead forms solid globules in imperfections throughout the alloy, such as
grain boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional crystallographic defect, defects in the crystal structure, and tend to decrease the ...
. In low concentrations, as well as acting as a lubricant, the globules hinder the formation of
swarf
Swarf, also known as chips or by other process-specific names (such as turnings, filings, or shavings), are pieces of metal, wood, or plastic that are the debris or waste resulting from machining, woodworking, or similar subtractive (material-r ...
as the alloy is worked, thereby improving machinability. Copper alloys with larger concentrations of lead are used in
bearings. The lead provides lubrication, and the copper provides the load-bearing support.
Lead's high density, atomic number, and formability form the basis for use of lead as a barrier that absorbs sound, vibration, and radiation. Lead has no natural resonance frequencies; as a result, sheet-lead is used as a sound deadening layer in the walls, floors, and ceilings of sound studios.
Organ pipe
An organ pipe is a sound-producing element of the pipe organ that resonator, resonates at a specific Pitch (music), pitch when pressurized air (commonly referred to as ''wind'') is driven through it. Each pipe is tuned to a note of the musical ...
s are often made from a lead alloy, mixed with various amounts of tin to control the tone of each pipe. Lead is an established
shielding material from
radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
* ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infr ...
in nuclear science and in X-ray rooms due to its denseness and high
attenuation coefficient
The linear attenuation coefficient, attenuation coefficient, or narrow-beam attenuation coefficient characterizes how easily a volume of material can be penetrated by a beam of light, sound, particles, or other energy or matter. A coefficient val ...
. Molten lead has been used as a coolant for
lead-cooled fast reactor
The lead-cooled fast reactor is a nuclear reactor design that uses molten lead or lead-bismuth eutectic as its coolant. These materials can be used as the primary coolant because they have low neutron absorption and relatively low melting poi ...
s.
Batteries
The largest use of lead in the early 21st century is in
leadŌĆōacid batteries. The lead in batteries undergoes no direct contact with humans, so there are fewer toxicity concerns. People who work in lead battery production or recycling plants may be exposed to lead dust and inhale it. The reactions in the battery between lead, lead dioxide, and sulfuric acid provide a reliable source of
voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
.
Supercapacitor
alt=Supercapacitor, upright=1.5, Schematic illustration of a supercapacitor
upright=1.5, A diagram that shows a hierarchical classification of supercapacitors and capacitors of related types
A supercapacitor (SC), also called an ultracapacitor, ...
s incorporating leadŌĆōacid batteries have been installed in kilowatt and megawatt scale applications in Australia, Japan, and the United States in frequency regulation, solar smoothing and shifting, wind smoothing, and other applications. These batteries have lower energy density and charge-discharge efficiency than
lithium-ion batteries
A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. Li-ion batteries are characterized by higher specific energy, energy ...
, but are significantly cheaper.
Coating for cables
Lead is used in high voltage power cables as shell material to prevent water diffusion into insulation; this use is decreasing as lead is being phased out. Its use in
solder
Solder (; North American English, NA: ) is a fusible alloy, fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces aft ...
for electronics is also being phased out by some countries to reduce the amount of
environmentally hazardous
Environmental hazards are hazards that affect biomes or ecosystems. Well known examples include oil spills, water pollution, slash and burn deforestation, air pollution, ground fissures, and Carbon dioxide in Earth's atmosphere, build-up of atmosp ...
waste. Lead is one of three metals used in the
Oddy test for museum materials, helping detect organic acids, aldehydes, acidic gases.
Compounds

In addition to being the main application for lead metal, leadŌĆōacid batteries are also the main consumer of lead compounds. The energy storage/release reaction used in these devices involves
lead sulfate
Lead(II) sulfate (PbSO4) is a white solid, which appears white in microcrystalline form. It is also known as ''fast white'', ''milk white'', ''sulfuric acid lead salt'' or ''anglesite''.
It is often seen in the plates/electrodes of car batteries ...
and
lead dioxide
Lead(IV) oxide, commonly known as lead dioxide, is an inorganic compound with the chemical formula . It is an oxide where lead is in an oxidation state of +4. It is a dark-brown solid which is insoluble in water. It exists in two crystalline forms ...
:
:(s) + (s) + 2(aq) ŌåÆ 2(s) + 2(l)
Other applications of lead compounds are very specialized and often fading. Lead-based coloring agents are used in
ceramic glaze
Ceramic glaze, or simply glaze, is a glassy coating on ceramics. It is used for decoration, to ensure the item is impermeable to liquids and to minimize the adherence of pollutants.
Glazing renders earthenware impermeable to water, sealing th ...
s and glass, especially for red and yellow shades. While lead paints are phased out in Europe and North America, they remain in use in less developed countries such as China, India, or Indonesia. Lead tetraacetate and lead dioxide are used as oxidizing agents in organic chemistry. Lead is frequently used in the
polyvinyl chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of ...
coating of electrical cords. It can be used to treat candle wicks to ensure a longer, more even burn. Because of its toxicity, European and North American manufacturers use alternatives such as zinc.
Lead glass
Lead glass, commonly called crystal, is a variety of glass in which lead replaces the calcium content of a typical potash glass. Lead glass contains typically 18ŌĆō40% (by mass) lead(II) oxide (PbO), while modern lead crystal, historically a ...
is composed of 12ŌĆō28%
lead oxide
Lead oxides are a group of inorganic compounds with formulas including lead (Pb) and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), gr ...
, changing its optical characteristics and reducing the transmission of ionizing radiation, a property used in old TVs and computer monitors with
cathode-ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
s. Lead-based
semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s such as
lead telluride
Lead telluride is a compound of lead (element), lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.3 ...
and
lead selenide
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. (Note that incorrectly identifies PbSe and ...
are used in
photovoltaic
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially ...
cells and
infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
detectors.
Biological effects
Lead has no confirmed biological role, and there is no confirmed safe level of lead exposure. A 2009 CanadianŌĆōAmerican study concluded that even at levels that are considered to pose little to no risk, lead may cause "adverse mental health outcomes". Its prevalence in the human bodyŌĆöat an adult average of 120 mgŌĆöis nevertheless exceeded only by zinc (2500 mg) and iron (4000 mg) among the heavy metals. Lead
salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
are very efficiently absorbed by the body. A small amount of lead (1%) is stored in bones; the rest is excreted in urine and feces within a few weeks of exposure. Only about a third of lead is excreted by a child. Continual exposure may result in the
bioaccumulation
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance faster than it can be lost or eliminated by catabolism and excretion. T ...
of lead.
Toxicity
Lead is a highly poisonous metal (whether inhaled or swallowed), affecting almost every organ and system in the human body. At airborne levels of 100 mg/m
3, it is
immediately dangerous to life and health. Most ingested lead is absorbed into the bloodstream. The primary cause of its toxicity is its predilection for interfering with the proper functioning of enzymes. It does so by binding to the
sulfhydryl groups found on many enzymes, or mimicking and displacing other metals which act as
cofactors in many enzymatic reactions. The essential metals that lead interacts with include calcium, iron, and zinc. High levels of calcium and iron tend to provide some protection from lead poisoning; low levels cause increased susceptibility.
Effects
Lead can cause severe damage to the brain and kidneys and, ultimately, death. By mimicking calcium, lead can cross the
bloodŌĆōbrain barrier
The bloodŌĆōbrain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system ...
. It degrades the
myelin
Myelin Sheath ( ) is a lipid-rich material that in most vertebrates surrounds the axons of neurons to insulate them and increase the rate at which electrical impulses (called action potentials) pass along the axon. The myelinated axon can be lik ...
sheaths of
neuron
A neuron (American English), neurone (British English), or nerve cell, is an membrane potential#Cell excitability, excitable cell (biology), cell that fires electric signals called action potentials across a neural network (biology), neural net ...
s, reduces their numbers, interferes with
neurotransmission
Neurotransmission (Latin: ''transmissio'' "passage, crossing" from ''transmittere'' "send, let through") is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron (the presynaptic neuron ...
routes, and decreases neuronal growth. In the human body, lead inhibits
porphobilinogen synthase
Aminolevulinic acid dehydratase (porphobilinogen synthase, or ALA dehydratase, or aminolevulinate dehydratase) is an enzyme () that in humans is encoded by the ''ALAD'' gene. Porphobilinogen synthase (or ALA dehydratase, or aminolevulinate dehydra ...
and
ferrochelatase
Protoporphyrin ferrochelatase (EC 4.98.1.1, formerly EC 4.99.1.1, or ferrochelatase; systematic name protoheme ferro-lyase (protoporphyrin-forming)) is an enzyme encoded by the ''FECH'' gene in humans. Ferrochelatase catalyses the eighth and ter ...
, preventing both
porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll.
The structure of the molecule can be described ...
formation and the incorporation of iron into
protoporphyrin IX
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not sol ...
, the final step in
heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
synthesis. This causes ineffective heme synthesis and
microcytic anemia.
Symptoms of lead poisoning include
nephropathy
Kidney disease, or renal disease, technically referred to as nephropathy, is damage to or disease of a kidney
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organ (anatomy), organs that are a multilobar, multipap ...
,
colic
Colic or cholic () is a form of pain that starts and stops abruptly. It occurs due to muscular contractions of a hollow tube (small and large intestine, gall bladder, ureter, etc.) in an attempt to relieve an obstruction by forcing content ou ...
-like abdominal pains, and possibly weakness in the fingers, wrists, or ankles. Small blood pressure increases, particularly in middle-aged and older people, may be apparent and can cause
anemia
Anemia (also spelt anaemia in British English) is a blood disorder in which the blood has a reduced ability to carry oxygen. This can be due to a lower than normal number of red blood cells, a reduction in the amount of hemoglobin availabl ...
. Several studies, mostly cross-sectional, found an association between increased lead exposure and decreased heart rate variability. In pregnant women, high levels of exposure to lead may cause miscarriage. Chronic, high-level exposure has been shown to reduce fertility in males.
In a child's developing brain, lead interferes with
synapse
In the nervous system, a synapse is a structure that allows a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or a target effector cell. Synapses can be classified as either chemical or electrical, depending o ...
formation in the
cerebral cortex
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. It is the largest site of Neuron, neural integration in the central nervous system, and plays ...
,
neurochemical A neurochemical is a small organic molecule or peptide that participates in neural activity. The science of neurochemistry studies the functions of neurochemicals.
Prominent neurochemicals
Neurotransmitters and neuromodulators
*Glutamate is th ...
development (including that of neurotransmitters), and the organization of
ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by Gating (electrophysiol ...
s. Early childhood exposure has been linked with an increased risk of sleep disturbances and excessive daytime drowsiness in later childhood. High blood levels are associated with delayed puberty in girls. The rise and fall in exposure to airborne lead from the combustion of tetraethyl lead in gasoline during the 20th century has been linked with historical increases and
decreases in crime levels.
Exposure sources
Lead exposure is a global issue since lead mining and smelting, and battery manufacturing, disposal, and
recycling
Recycling is the process of converting waste materials into new materials and objects. This concept often includes the recovery of energy from waste materials. The recyclability of a material depends on its ability to reacquire the propert ...
, are common in many countries. Lead enters the body via inhalation, ingestion, or skin absorption. Almost all inhaled lead is absorbed into the body; for ingestion, the rate is 20ŌĆō70%, with children absorbing a higher percentage than adults.
Poisoning typically results from ingestion of food or water contaminated with lead, and less commonly after accidental ingestion of contaminated soil, dust, or lead-based paint. Seawater products can contain lead if affected by nearby industrial waters. Fruit and vegetables can be contaminated by high levels of lead in the soils they were grown in. Soil can be contaminated through particulate accumulation from lead in pipes,
lead paint
Lead paint or lead-based paint is paint containing lead. As pigment, lead(II) chromate (, "chrome yellow"), lead(II,IV) oxide, (, "red lead"), and lead(II) carbonate (, "white lead") are the most common forms.. Lead is added to paint to acceler ...
, residual emissions from leaded gasoline.
The use of lead for water pipes is
a problem in areas with soft or acidic water. Hard water forms insoluble protective layers on the inner surface of the pipes, whereas soft and acidic water dissolves the lead pipes. Dissolved
carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
in the carried water may result in the formation of soluble lead
bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
; oxygenated water may similarly dissolve lead as
lead(II) hydroxide. Drinking such water, over time, can cause health problems due to the toxicity of the dissolved lead. The
harder the water the more
calcium bicarbonate
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (), and carbonate () io ...
and
sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
it contains, and the more the inside of the pipes are coated with a protective layer of lead carbonate or lead sulfate.

Ingestion of applied lead-based paint is the major source of exposure for children: a direct source is chewing on old painted window sills. Additionally, as lead paint on a surface deteriorates, it peels and is pulverized into dust. The dust then enters the body through hand-to-mouth contact or contaminated food or drink. Ingesting certain
home remedies may result in exposure to lead or its compounds.
Inhalation is the second major exposure pathway, affecting smokers and especially workers in lead-related occupations.
Cigarette smoke contains, among other toxic substances, radioactive
lead-210
Lead (82Pb) has four observationally stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay ch ...
. "As a result of EPA's regulatory efforts, levels of lead in the air
n the United Statesdecreased by 86 percent between 2010 and 2020."
The concentration of lead in the air in the United States fell below the national standard of 0.15 ╬╝g/m
3 in 2014.
Skin exposure may be significant for people working with organic lead compounds. The rate of skin absorption is lower for inorganic lead.
Lead in foods
Lead may be found in food when food is grown in soil that is high in lead, airborne lead contaminates the crops, animals eat lead in their diet, or lead enters the food either from what it was stored or cooked in. Ingestion of lead paint and batteries is also a route of exposure for livestock, which can subsequently affect humans. Milk produced by contaminated cattle can be diluted to a lower lead concentration and sold for consumption.
In Bangladesh, lead compounds have been added to
turmeric
Turmeric (), or ''Curcuma longa'' (), is a flowering plant in the ginger family Zingiberaceae. It is a perennial, rhizomatous, herbaceous plant native to the Indian subcontinent and Southeast Asia that requires temperatures between and high ...
to make it more yellow.
This is believed to have started in the 1980s and continues .
[ It is believed to be one of the main sources of high lead levels in the country. In Hong Kong the maximum allowed lead level in food is 6 parts per million in solids and 1 part per million in liquids.
Lead-containing dust can settle on drying cocoa beans when they are set outside near polluting industrial plants. In December 2022, ]Consumer Reports
Consumer Reports (CR), formerly Consumers Union (CU), is an American nonprofit consumer organization dedicated to independent product testing, investigative journalism, consumer-oriented research, public education, and consumer advocacy.
Founded ...
tested 28 dark chocolate
Dark chocolate is a form of chocolate made from cocoa solids, cocoa butter and sugar. It has a higher cocoa percentage than white chocolate, milk chocolate, and semisweet chocolate. Dark chocolate is valued for claimedŌĆöthough unsupportedŌĆö ...
brands and found that 23 of them contained potentially harmful levels of lead, cadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
or both. They have urged the chocolate makers to reduce the level of lead which could be harmful, especially to a developing fetus.
In March 2024, the US Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
recommended a voluntary recall on 6 brands of cinnamon
Cinnamon is a spice obtained from the inner bark of several tree species from the genus ''Cinnamomum''. Cinnamon is used mainly as an aromatic condiment and flavouring additive in a wide variety of cuisines, sweet and savoury dishes, biscuits, b ...
due to contamination with lead, after 500 reports of child lead poisoning. The FDA determined that cinnamon was adulterated with lead chromate
Lead(II) chromate is an inorganic compound with the chemical formula . It is a bright yellow salt that is very poorly soluble in water. It occurs also as the mineral crocoite. It is used as a pigment.
Structure
Two polymorphs of lead chromat ...
.
Lead in plastic toys
According to the United States Center for Disease Control
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency under the Department of Health and Human Services (HHS), and is headquartered in Atlanta, ...
, the use of lead in plastics has not been banned as of 2024. Lead softens the plastic and makes it more flexible so that it can go back to its original shape. Habitual chewing on colored plastic insulation from stripped electrical wires was found to cause elevated lead levels in a 46-year-old man. Lead may be used in plastic toys to stabilize molecules from heat. Lead dust can be formed when plastic is exposed to sunlight, air, and detergents that break down the chemical bond between the lead and plastics.
Treatment
Treatment for lead poisoning normally involves the administration of dimercaprol
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead. It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is n ...
and succimer
Succimer, sold under the brand name Chemet among others, is a medication tool used to treat lead, mercury, and arsenic poisoning. When it's radiolabeled with technetium-99m, it's used in many types of diagnostic testing.
Common side effe ...
. Acute cases may require the use of disodium calcium edetate, the calcium chelate
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
, and the disodium salt of ethylenediaminetetraacetic acid (EDTA
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula . This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-solubl ...
). It has a greater affinity for lead than calcium, with the result that lead chelate is formed by exchange and excreted in the urine, leaving behind harmless calcium.
Environmental effects
 The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting
The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
and at high enough concentrations, negatively affecting plant growth and survival. Contamination of soils and plants can allow lead to ascend the food chain affecting microorganisms and animals. In animals, lead exhibits toxicity in many organs, damaging the nervous, renal
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and right in the retrop ...
, reproductive, hematopoietic
Haematopoiesis (; ; also hematopoiesis in American English, sometimes h(a)emopoiesis) is the formation of blood cellular components. All cellular blood components are derived from haematopoietic stem cells. In a healthy adult human, roughly ten ...
, and cardiovascular systems after ingestion, inhalation, or skin absorption. Fish uptake lead from both water and sediment; bioaccumulation in the food chain poses a hazard to fish, birds, and sea mammals.
Anthropogenic lead includes lead from shot
Shot may refer to:
Arts, entertainment, and media
* ''Shot'' (album), by The Jesus Lizard
*''Shot, Illusion, New God'', an EP by Gruntruck
*'' Shot Rev 2.0'', a video album by The Sisters of Mercy
* "Shot" (song), by The Rasmus
* ''Shot'' (2017 ...
and sinkers. These are among the most potent sources of lead contamination along with lead production sites. Lead was banned for shot and sinkers in the United States in 2017, although that ban was only effective for a month, and a similar ban is being considered in the European Union.
Analytical methods for the determination of lead in the environment include spectrophotometry
Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spe ...
, X-ray fluorescence
X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis ...
, atomic spectroscopy
In physics, atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Since unique elements have unique emission spectra, atomic spectroscopy is applied for determination of elemental compositions. It can ...
, and electrochemical methods. A specific ion-selective electrode has been developed based on the ionophore ''S'',''S-methylenebis(''N'',''N''-diisobutyldithiocarbamate
In organic chemistry, a dithiocarbamate is a chemical compound with the general formula . It contains the functional group with the Chemical structure, structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur ...
). An important biomarker assay for lead poisoning is ╬┤-aminolevulinic acid
╬┤-Aminolevulinic acid (also dALA, ╬┤-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyl ...
levels in plasma, serum, and urine.
Restriction and remediation
By the mid-1980s, there was significant decline in the use of lead in industry. In the United States, environmental regulations reduced or eliminated the use of lead in non-battery products, including gasoline, paints, solders, and water systems. Particulate control devices were installed in coal-fired power plants
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is a type of ...
to capture lead emissions. In 1992, U.S. Congress required the Environmental Protection Agency to reduce the blood lead levels of the country's children. Lead use was further curtailed by the European Union's 2003 Restriction of Hazardous Substances Directive
The Restriction of Hazardous Substances Directive 2002/95/EC (RoHS 1), short for Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment, was adopted in February 2003 by the European Uni ...
. A large drop in lead deposition occurred in the Netherlands after the 1993 national ban on use of lead shot for hunting and sport shooting: from 230 tonnes in 1990 to 47.5 tonnes in 1995. The usage of lead in Avgas 100LL for general aviation
General aviation (GA) is defined by the International Civil Aviation Organization (ICAO) as all civil aviation aircraft operations except for commercial air transport or aerial work, which is defined as specialized aviation services for other ...
is allowed in the EU as of 2022.permissible exposure limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agents such as high level noise. Permissible exposure limits were established by the Occupational ...
for lead in the workplace, comprising metallic lead, inorganic lead compounds, and lead soaps, was set at 50 ╬╝g/m3 over an 8-hour workday, and the blood lead level
Blood lead level (BLL), is a measure of the amount of lead in the blood. Lead is a toxic heavy metal and can cause neurological damage, especially among children, at any detectable level. High lead levels cause decreased vitamin D and haemo ...
limit at 5 ╬╝g per 100 g of blood in 2012. Lead may still be found in harmful quantities in stoneware, vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
(such as that used for tubing and the insulation of electrical cords), and Chinese brass. Old houses may still contain lead paint. White lead paint has been withdrawn from sale in industrialized countries, but specialized uses of other pigments such as yellow lead chromate
Lead(II) chromate is an inorganic compound with the chemical formula . It is a bright yellow salt that is very poorly soluble in water. It occurs also as the mineral crocoite. It is used as a pigment.
Structure
Two polymorphs of lead chromat ...
remain, especially in road pavement marking paint.
Stripping old paint by sanding produces dust which can be inhaled. Lead abatement
Lead abatement includes lead-based paint wiktionary:abatement, abatement activities, such as inspections, risk assessments, as well as removal. Lead abatement must be performed by educated, certified professionals with proper safety protocols to ...
programs have been mandated by some authorities in properties where young children live. The usage of lead in Avgas 100LL for general aviation
General aviation (GA) is defined by the International Civil Aviation Organization (ICAO) as all civil aviation aircraft operations except for commercial air transport or aerial work, which is defined as specialized aviation services for other ...
is generally allowed in United States as of 2023.
Lead waste, depending on the jurisdiction and the nature of the waste, may be treated as household waste (to facilitate lead abatement activities), or potentially hazardous waste requiring specialized treatment or storage. Lead is released into the environment in shooting places and a number of lead management practices have been developed to counter the lead contamination. Lead migration can be enhanced in acidic soils; to counter that, it is advised soils be treated with lime to neutralize the soils and prevent leaching of lead.
Research has been conducted on how to remove lead from biosystems by biological means: Fish bones are being researched for their ability to bioremediate
Bioremediation broadly refers to any process wherein a biological system (typically bacteria, microalgae, fungi in mycoremediation, and plants in phytoremediation), living or dead, is employed for removing environmental pollutants from air, wat ...
lead in contaminated soil. The fungus '' Aspergillus versicolor'' is effective at absorbing lead ions from industrial waste before being released to water bodies. Several bacteria have been researched for their ability to remove lead from the environment, including the sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termina ...
''Desulfovibrio
''Desulfovibrio'' is a genus of Gram-negative sulfate-reducing bacteria. ''Desulfovibrio'' species are commonly found in aquatic environments with high levels of organic material, as well as in water-logged soils, and form major community member ...
'' and ''Desulfotomaculum
''Desulfotomaculum'' is a genus of Gram-positive, obligately anaerobic soil bacteria. A type of sulfate-reducing bacteria, ''Desulfotomaculum'' can cause food spoilage in poorly processed canned foods. Their presence can be identified by the re ...
'', both of which are highly effective in aqueous solutions. Millet grass '' Urochloa ramosa'' has the ability to accumulate significant amounts of metals such as lead and zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
in its shoot and root tissues making it an important plant for remediation of contaminated soils.
See also
* Derek Bryce-Smith ŌĆō one of the earliest campaigners against lead in petrol in the UK
* Thomas Midgley Jr. ŌĆō discovered that the addition of tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
to gasoline prevented "knocking" in internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal comb ...
s
* Clair Cameron Patterson ŌĆō instrumental in the banning of tetraethyllead in gasoline in the US and lead solder in food cans.
* Robert A. Kehoe ŌĆō foremost medical advocate for the use of tetraethyllead as an additive in gasoline.
Notes
References
Bibliography
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Further reading
*
Table of contents
*
*
* Ingalls, Walter Renton (1865-)
Lead Smelting and Refining, With Some Notes on Lead Mining
*
External links
The Toxicology of Heavy Metals: Getting the Lead Out
American Society for Clinical Pathology
The American Society for Clinical Pathology (ASCP), formerly known as the American Society of Clinical Pathologists, is a professional association based in Chicago, Illinois, encompassing 130,000 pathologists and laboratory professionals.
Founded ...
COVID-19 to reduce global lead production by 5,2% in 2020 (with a figure showing global lead production, 2010ŌĆō2024)
{{Authority control
Chemical elements
Hazardous materials
Post-transition metals
Native element minerals
Superconductors
Endocrine disruptors
IARC Group 2B carcinogens
Nuclear reactor coolants
Chemical elements with face-centered cubic structure
 The melting point of leadŌĆöat 327.5 ┬░C (621.5 ┬░F)ŌĆöis very low compared to most metals. Its
The melting point of leadŌĆöat 327.5 ┬░C (621.5 ┬░F)ŌĆöis very low compared to most metals. Its  Three of the stable isotopes are found in three of the four major
Three of the stable isotopes are found in three of the four major  Lead dihalides are well-characterized; this includes the diastatide and mixed halides, such as PbFCl. The relative insolubility of the latter forms a useful basis for the gravimetric determination of fluorine. The difluoride was the first solid ionically conducting compound to be discovered (in 1834, by
Lead dihalides are well-characterized; this includes the diastatide and mixed halides, such as PbFCl. The relative insolubility of the latter forms a useful basis for the gravimetric determination of fluorine. The difluoride was the first solid ionically conducting compound to be discovered (in 1834, by  Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained, as an intermediate between lead(II) and lead(IV), in larger organolead complexes; this oxidation state is not stable, as both the lead(III) ion and the larger complexes containing it are
Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained, as an intermediate between lead(II) and lead(IV), in larger organolead complexes; this oxidation state is not stable, as both the lead(III) ion and the larger complexes containing it are  Lead can form multiply-bonded chains, a property it shares with its lighter
Lead can form multiply-bonded chains, a property it shares with its lighter  Rome's territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the
Rome's territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the  Lead tablets were commonly used as a material for letters. Lead coffins, cast in flat sand forms and with interchangeable motifs to suit the faith of the deceased, were used in ancient
Lead tablets were commonly used as a material for letters. Lead coffins, cast in flat sand forms and with interchangeable motifs to suit the faith of the deceased, were used in ancient  In the second half of the 18th century, Britain, and later continental Europe and the United States, experienced the
In the second half of the 18th century, Britain, and later continental Europe and the United States, experienced the  Further evidence of the threat that lead posed to humans was discovered in the late 19th and early 20th centuries. Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe. The United Kingdom introduced mandatory factory inspections in 1878 and appointed the first Medical Inspector of Factories in 1898; as a result, a 25-fold decrease in lead poisoning incidents from 1900 to 1944 was reported. Most European countries banned lead paintŌĆöcommonly used because of its opacity and water resistanceŌĆöfor interiors by 1930.
The last major human exposure to lead was the addition of
Further evidence of the threat that lead posed to humans was discovered in the late 19th and early 20th centuries. Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe. The United Kingdom introduced mandatory factory inspections in 1878 and appointed the first Medical Inspector of Factories in 1898; as a result, a 25-fold decrease in lead poisoning incidents from 1900 to 1944 was reported. Most European countries banned lead paintŌĆöcommonly used because of its opacity and water resistanceŌĆöfor interiors by 1930.
The last major human exposure to lead was the addition of  Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. When the pencil originated as a wrapped graphite writing tool, the particular type of
Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. When the pencil originated as a wrapped graphite writing tool, the particular type of  In addition to being the main application for lead metal, leadŌĆōacid batteries are also the main consumer of lead compounds. The energy storage/release reaction used in these devices involves
In addition to being the main application for lead metal, leadŌĆōacid batteries are also the main consumer of lead compounds. The energy storage/release reaction used in these devices involves  Ingestion of applied lead-based paint is the major source of exposure for children: a direct source is chewing on old painted window sills. Additionally, as lead paint on a surface deteriorates, it peels and is pulverized into dust. The dust then enters the body through hand-to-mouth contact or contaminated food or drink. Ingesting certain home remedies may result in exposure to lead or its compounds.
Inhalation is the second major exposure pathway, affecting smokers and especially workers in lead-related occupations. Cigarette smoke contains, among other toxic substances, radioactive
Ingestion of applied lead-based paint is the major source of exposure for children: a direct source is chewing on old painted window sills. Additionally, as lead paint on a surface deteriorates, it peels and is pulverized into dust. The dust then enters the body through hand-to-mouth contact or contaminated food or drink. Ingesting certain home remedies may result in exposure to lead or its compounds.
Inhalation is the second major exposure pathway, affecting smokers and especially workers in lead-related occupations. Cigarette smoke contains, among other toxic substances, radioactive  The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting
The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting