Isotopes Of Hydrogen on:
[Wikipedia]
[Google]
[Amazon]

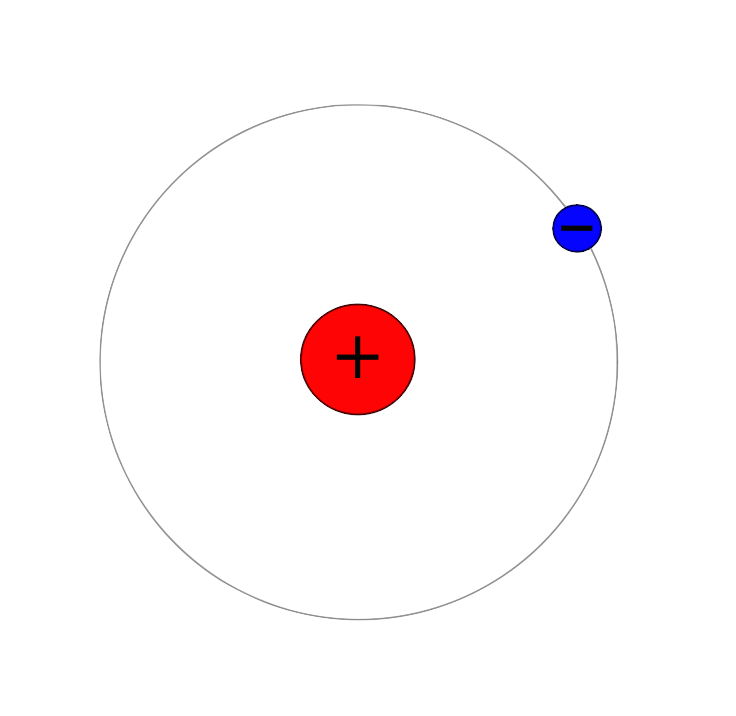 H (atomic mass ) is the most common hydrogen isotope, with an abundance of >99.98%. Its nucleus consists of only a single
H (atomic mass ) is the most common hydrogen isotope, with an abundance of >99.98%. Its nucleus consists of only a single
 ''Deuterium'', H (atomic mass ), the other stable hydrogen isotope, has one proton and one neutron in its nucleus, called a deuteron. H comprises 26–184 ppm (by population, not mass) of hydrogen on Earth; the lower number tends to be found in hydrogen gas and higher enrichment (150 ppm) is typical of
''Deuterium'', H (atomic mass ), the other stable hydrogen isotope, has one proton and one neutron in its nucleus, called a deuteron. H comprises 26–184 ppm (by population, not mass) of hydrogen on Earth; the lower number tends to be found in hydrogen gas and higher enrichment (150 ppm) is typical of
 ''Tritium'', H (atomic mass ), has one proton and two neutrons in its nucleus (triton). It is radioactive, β decaying into helium-3 with
''Tritium'', H (atomic mass ), has one proton and two neutrons in its nucleus (triton). It is radioactive, β decaying into helium-3 with
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
(H) has three naturally occurring isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s: H, H, and H. H and H are stable, while H has a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of years. Heavier isotopes also exist; all are synthetic and have a half-life of less than 1 zeptosecond (10 s).
Of these, H is the least stable, while H is the most.
Hydrogen is the only element whose isotopes have different names that remain in common use today: H is deuterium and H is tritium. The symbols D and T are sometimes used for deuterium and tritium; IUPAC (International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
) accepts said symbols, but recommends the standard isotopic symbols H and H, to avoid confusion in alphabetic sorting of chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
s. H, with no neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s, may be called protium to disambiguate. (During the early study of radioactivity, some other heavy radioisotopes were given names
A name is a term used for identification by an external observer. They can identify a class or category of things, or a single thing, either uniquely, or within a given context. The entity identified by a name is called its referent. A person ...
, but such names are rarely used today.)
List of isotopes
Note: "y" means year, but "ys" means yoctosecond (10 second). , - , H , 1 , 0 , , colspan=3 align=center, StableUnless proton decay occurs.This and He are the only stable nuclides with more protons than neutrons. , 1/2+ , colspan="2" style="text-align:center" , ref name="Atomic Weight of Hydrogen"> , Protium , - , H (D)Produced in Big Bang nucleosynthesis.One of the few stable odd-odd nuclei , 1 , 1 , , colspan=3 align=center , Stable , 1+ , colspan="2" style="text-align:center" , ref name="Atomic Weight of Hydrogen" /> , Deuterium , - , H (T) , 1 , 2 , , , β , He , 1/2+ , TraceTritium occurs naturally as a cosmogenic nuclide. , , Tritium , - , H , 1 , 3 , , , n , H , 2− , , , - , H , 1 , 4 , , , 2n , H , (1/2+) , , , - , H , 1 , 5 , , , , , 2−# , , , - , H , 1 , 6 , # , , , , 1/2+# , ,Hydrogen-1 (protium)
 H (atomic mass ) is the most common hydrogen isotope, with an abundance of >99.98%. Its nucleus consists of only a single
H (atomic mass ) is the most common hydrogen isotope, with an abundance of >99.98%. Its nucleus consists of only a single proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
, so it has the formal name protium.
The proton has never been observed to decay, so H is considered stable. It is the only stable nuclide with no neutrons. Some Grand Unified Theories proposed in the 1970s predict that proton decay can occur with a half-life between and years. If so, then H (and all nuclei now believed to be stable) are only observationally stable. As of 2018, experiments have shown that the mean lifetime of the proton is > years.
Hydrogen-2 (deuterium)
 ''Deuterium'', H (atomic mass ), the other stable hydrogen isotope, has one proton and one neutron in its nucleus, called a deuteron. H comprises 26–184 ppm (by population, not mass) of hydrogen on Earth; the lower number tends to be found in hydrogen gas and higher enrichment (150 ppm) is typical of
''Deuterium'', H (atomic mass ), the other stable hydrogen isotope, has one proton and one neutron in its nucleus, called a deuteron. H comprises 26–184 ppm (by population, not mass) of hydrogen on Earth; the lower number tends to be found in hydrogen gas and higher enrichment (150 ppm) is typical of seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
. Deuterium on Earth has been enriched with respect to its initial concentration in the Big Bang and outer Solar System (≈27 ppm, atom fraction) and older parts of the Milky Way
The Milky Way or Milky Way Galaxy is the galaxy that includes the Solar System, with the name describing the #Appearance, galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars in other arms of the galax ...
(≈23 ppm). Presumably the differential concentration of deuterium in the inner Solar System is due to the lower volatility of deuterium gas and compounds, enriching deuterium fractions in comet
A comet is an icy, small Solar System body that warms and begins to release gases when passing close to the Sun, a process called outgassing. This produces an extended, gravitationally unbound atmosphere or Coma (cometary), coma surrounding ...
s and planets exposed to significant heat from the Sun over billions of years of Solar System evolution.
Deuterium is not radioactive, and is not a significant toxicity hazard. Water enriched in H is called heavy water
Heavy water (deuterium oxide, , ) is a form of water (molecule), water in which hydrogen atoms are all deuterium ( or D, also known as ''heavy hydrogen'') rather than the common hydrogen-1 isotope (, also called ''protium'') that makes up most o ...
. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for H- nuclear magnetic resonance spectroscopy. Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
.
Hydrogen-3 (tritium)
 ''Tritium'', H (atomic mass ), has one proton and two neutrons in its nucleus (triton). It is radioactive, β decaying into helium-3 with
''Tritium'', H (atomic mass ), has one proton and two neutrons in its nucleus (triton). It is radioactive, β decaying into helium-3 with half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
. Traces of H occur naturally due to cosmic rays interacting with atmospheric gases. H has also been released in nuclear tests. It is used in fusion bombs, as a tracer in isotope geochemistry, and in self-powered lighting
Tritium radioluminescence is the use of gaseous tritium, a radioactive isotope of hydrogen, to create visible light. Tritium emits electrons through beta decay and, when they interact with a phosphor material, light is emitted through the proc ...
devices.
The most common way to produce H is to bombard a natural isotope of lithium, Li, with neutrons in a nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
.
Tritium can be used in chemical and biological labeling experiments as a radioactive tracer. Deuterium–tritium fusion uses H and H as its main reactants, giving energy through the loss of mass when the two nuclei collide and fuse at high temperatures.
Hydrogen-4
H ( atomic mass ), with one proton and three neutrons, is a highlyunstable
In dynamical systems instability means that some of the outputs or internal state (controls), states increase with time, without bounds. Not all systems that are not Stability theory, stable are unstable; systems can also be marginal stability ...
isotope. It has been synthesized in the laboratory by bombarding tritium with fast-moving deuterons;
the triton captured a neutron from the deuteron. The presence of H was deduced by detecting the emitted protons. It decays by neutron emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisin ...
into H with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of (or ).
In the 1955 satirical novel '' The Mouse That Roared'', the name quadium was given to the H that powered the ''Q-bomb'' that the Duchy of Grand Fenwick captured from the United States.
Hydrogen-5
H ( atomic mass ), with one proton and four neutrons, is highly unstable. It has been synthesized in the lab by bombarding tritium with fast-moving tritons; one triton captures two neutrons from the other, becoming a nucleus with one proton and four neutrons. The remaining proton may be detected, and the existence of H deduced. It decays by doubleneutron emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisin ...
into H and has a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of () – the shortest half-life of any known nuclide.
Hydrogen-6
H ( atomic mass ) has oneproton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
and five neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s. It has a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of (). In 2025, ⁶H was produced using an 855MeV electron beam impinging upon on a ⁷Li target.
Hydrogen-7
H ( atomic mass ) has oneproton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
and six neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s. It was first synthesized in 2003 by a group of Russian, Japanese and French scientists at Riken's Radioactive Isotope Beam Factory by bombarding hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
with helium-8 atoms; all six of the helium-8's neutrons were donated to the hydrogen nucleus. The two remaining protons were detected by the "Riken telescope", a device made of several layers of sensors, positioned behind the target of the RI Beam cyclotron. H has a half-life of ().
Decay chains
H and H decay directly to H, which then decays to stable He. Decay of the heaviest isotopes, H and H, has not been experimentally observed. : Decay times are in yoctoseconds () for all these isotopes except H, which is in years.See also
* Hydrogen atom * Hydrogen isotope biogeochemistry * Hydrogen-4.1 (Muonic helium) * Muonium – acts like an exotic light isotope of hydrogen *Notes
References
Further reading
* {{Authority control HydrogenHydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...