isotonic fluid on:
[Wikipedia]
[Google]
[Amazon]

 In
In
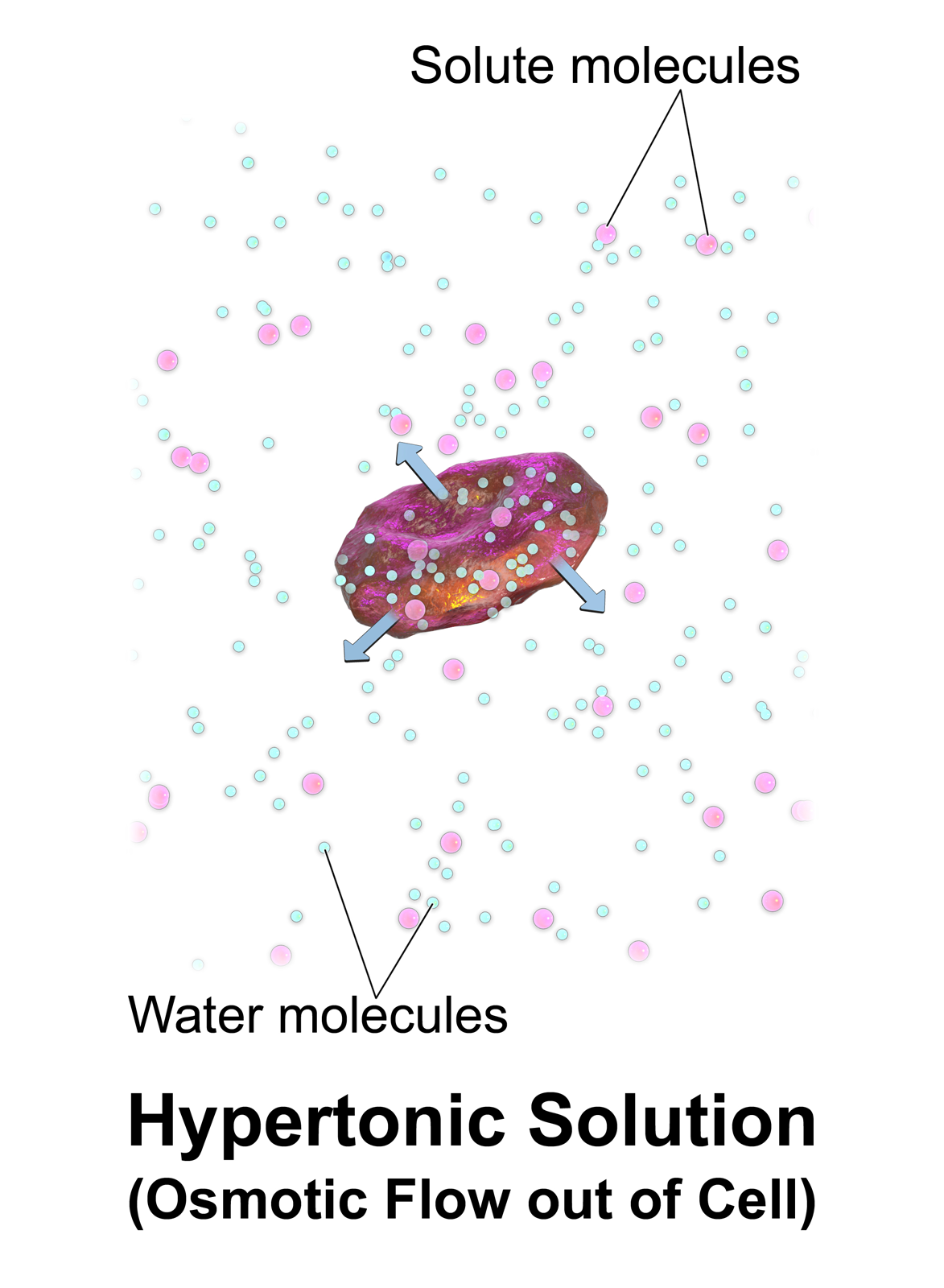 A hypertonic solution has a greater concentration of non-permeating
A hypertonic solution has a greater concentration of non-permeating
 A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to the
A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to the
 A solution is isotonic when its effective
A solution is isotonic when its effective
chemical biology
Chemical biology is a scientific discipline between the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and m ...
, tonicity is a measure of the effective osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a Solution (chemistry), solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a soluti ...
gradient; the water potential
Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure and mat ...
of two solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Solu ...
s separated by a partially-permeable cell membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
. Tonicity depends on the relative concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
of selective membrane-impermeable solutes
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are ...
across a cell membrane which determine the direction and extent of osmotic flux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications in physics. For transport phe ...
. It is commonly used when describing the swelling-versus-shrinking response of cells
Cell most often refers to:
* Cell (biology), the functional basic unit of life
* Cellphone, a phone connected to a cellular network
* Clandestine cell, a penetration-resistant form of a secret or outlawed organization
* Electrochemical cell, a d ...
immersed in an external solution.
Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition
Imbibition is a special type of diffusion that takes place when liquid is absorbed by solids-Colloid, colloids causing an increase in volume. Water surface potential movement takes place along a concentration gradient; some dry materials absorb ...
.
There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''. A hypotonic solution example is distilled water.
Hypertonic solution
 A hypertonic solution has a greater concentration of non-permeating
A hypertonic solution has a greater concentration of non-permeating solute
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are ...
s than another solution. In biology, the tonicity of a solution usually refers to its solute concentration relative to that of another solution on the opposite side of a cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
; a solution outside of a cell is called hypertonic if it has a greater concentration of solutes than the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
inside the cell. When a cell is immersed in a hypertonic solution, osmotic pressure tends to force water to flow out of the cell in order to balance the concentrations of the solutes on either side of the cell membrane. The cytosol is conversely categorized as hypotonic, opposite of the outer solution.
When plant cells are in a hypertonic solution, the flexible cell membrane pulls away from the rigid cell wall
A cell wall is a structural layer that surrounds some Cell type, cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, ...
, but remains joined to the cell wall at points called plasmodesmata
Plasmodesmata (singular: plasmodesma) are microscopic channels which traverse the cell walls of plant cells and some algal cells, enabling transport and communication between them. Plasmodesmata evolved independently in several lineages, and spe ...
. The cells often take on the appearance of a pincushion
A pincushion (or pin cushion) is a small, stuffed cushion, typically across, which is used in sewing to store pins or Sewing needle, needles with their heads protruding to take hold of them easily, collect them, and keep them organized.
Pincus ...
, and the plasmodesmata almost cease to function because they become constricted, a condition known as plasmolysis
Plasmolysis is the process in which cells lose water in a hypertonic solution. The reverse process, deplasmolysis or cytolysis, can occur if the cell is in a hypotonic solution resulting in a lower external osmotic pressure and a net flow of wa ...
. In plant cells the terms isotonic, hypotonic and hypertonic cannot strictly be used accurately because the pressure exerted by the cell wall significantly affects the osmotic equilibrium point.
Some organisms have evolved intricate methods of circumventing hypertonicity. For example, saltwater is hypertonic to the fish
A fish (: fish or fishes) is an aquatic animal, aquatic, Anamniotes, anamniotic, gill-bearing vertebrate animal with swimming fish fin, fins and craniate, a hard skull, but lacking limb (anatomy), limbs with digit (anatomy), digits. Fish can ...
that live in it. Because the fish need a large surface area in their gill
A gill () is a respiration organ, respiratory organ that many aquatic ecosystem, aquatic organisms use to extract dissolved oxygen from water and to excrete carbon dioxide. The gills of some species, such as hermit crabs, have adapted to allow r ...
s in contact with seawater for gas exchange
Gas exchange is the physical process by which gases move passively by diffusion across a surface. For example, this surface might be the air/water interface of a water body, the surface of a gas bubble in a liquid, a gas-permeable membrane, or a b ...
, they lose water osmotically to the sea from gill cells. They respond to the loss by drinking large amounts of saltwater, and actively excreting the excess salt. This process is called osmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration ...
.
Hypotonic solution
 A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to the
A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
. Due to osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a Solution (chemistry), solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a soluti ...
, water diffuses into the cell, and the cell often appears turgid
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall.
It is also called ''hydrostatic pressure'', and is defined as the pressure in a fluid measured at a certain point within itself when at equilibri ...
, or bloated.
For cells without a cell wall
A cell wall is a structural layer that surrounds some Cell type, cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, ...
such as animal cells, if the gradient is large enough, the uptake of excess water can produce enough pressure to induce cytolysis
Cytolysis, or osmotic lysis, occurs when a cell bursts due to an osmotic imbalance that has caused excess water to diffuse into the cell. Water can enter the cell by diffusion through the cell membrane or through selective membrane channels ...
, or rupturing of the cell.
When plant cells are in a hypotonic solution, the central vacuole
A vacuole () is a membrane-bound organelle which is present in Plant cell, plant and Fungus, fungal Cell (biology), cells and some protist, animal, and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water ...
takes on extra water and pushes the cell membrane against the cell wall. Due to the rigidity of the cell wall, it pushes back, preventing the cell from bursting. This is called turgor pressure
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall.
It is also called ''hydrostatic pressure'', and is defined as the pressure in a fluid measured at a certain point within itself when at equilibri ...
.
Isotonicity
 A solution is isotonic when its effective
A solution is isotonic when its effective osmole
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of wiktionary:osmole, osmoles (Osm) of solute per litre (L) of Solution (chemistry), solution (osmol/L or Osm/L). The osmolarity of ...
concentration is the same as that of another solution. In biology, the solutions on either side of a cell membrane are isotonic if the concentration of solutes outside the cell is equal to the concentration of solutes inside the cell. In this case the cell neither swells nor shrinks because there is no concentration gradient to induce the diffusion of large amounts of water across the cell membrane. Water molecules freely diffuse through the plasma membrane in both directions, and as the rate of water diffusion is the same in each direction, the cell will neither gain nor lose water.
An iso-osmolar solution can be hypotonic if the solute is able to penetrate the cell membrane. For example, an iso-osmolar urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
solution is hypotonic to red blood cells, causing their lysis
Lysis ( ; from Greek 'loosening') is the breaking down of the membrane of a cell, often by viral, enzymic, or osmotic (that is, "lytic" ) mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a ...
. This is due to urea entering the cell down its concentration gradient, followed by water. The osmolarity of normal saline
Saline (also known as saline solution) is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By intravenous therapy, inje ...
, 9 grams NaCl
Sodium chloride , commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral hali ...
dissolved in water to a total volume of one liter, is a close approximation to the osmolarity of NaCl in blood (about 290 mOsm/ L). Thus, normal saline is almost isotonic to blood plasma. Neither sodium nor chloride ions can freely pass through the plasma membrane, unlike urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
.
See also
*Osmotic concentration
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/ ...
*Osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively permeable membrane, selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of ...
*Salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensio ...
References
{{reflist Cell biology