
Heat transfer is a discipline of
thermal engineering that concerns the generation, use, conversion, and exchange of
thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
(
heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
) between physical systems. Heat transfer is classified into various mechanisms, such as
thermal conduction
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy ...
,
thermal convection,
thermal radiation, and transfer of energy by
phase changes. Engineers also consider the transfer of mass of differing chemical species (mass transfer in the form of
advection), either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
Heat conduction, also called diffusion, is the direct microscopic exchanges of kinetic energy of particles (such as molecules) or quasiparticles (such as lattice waves) through the boundary between two systems. When an object is at a different
temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
from another body or its surroundings,
heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
flows so that the body and the surroundings reach the same temperature, at which point they are in
thermal equilibrium. Such spontaneous heat transfer always occurs from a region of high temperature to another region of lower temperature, as described in the
second law of thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spont ...
.
Heat convection occurs when the bulk flow of a fluid (gas or liquid) carries its heat through the fluid. All convective processes also move heat partly by diffusion, as well. The flow of fluid may be forced by external processes, or sometimes (in gravitational fields) by buoyancy forces caused when thermal energy expands the fluid (for example in a fire plume), thus influencing its own transfer. The latter process is often called "natural convection". The former process is often called "forced convection." In this case, the fluid is forced to flow by use of a pump, fan, or other mechanical means.
Thermal radiation occurs through a
vacuum or any
transparent medium (
solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
or
fluid
In physics, a fluid is a liquid, gas, or other material that may continuously motion, move and Deformation (physics), deform (''flow'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are M ...
or
gas). It is the transfer of energy by means of
photons or
electromagnetic waves
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength, ran ...
governed by the same laws.
Overview

Heat transfer is the energy exchanged between materials (solid/liquid/gas) as a result of a temperature difference. The
thermodynamic free energy
In thermodynamics, the thermodynamic free energy is one of the state functions of a thermodynamic system. The change in the free energy is the maximum amount of work that the system can perform in a process at constant temperature, and its ...
is the amount of work that a thermodynamic system can perform.
Enthalpy is a
thermodynamic potential
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
, designated by the letter "H", that is the sum of the
internal energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accoun ...
of the system (U) plus the product of
pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
(P) and
volume
Volume is a measure of regions in three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch) ...
(V).
Joule
The joule ( , or ; symbol: J) is the unit of energy in the International System of Units (SI). In terms of SI base units, one joule corresponds to one kilogram- metre squared per second squared One joule is equal to the amount of work d ...
is a unit to quantify
energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
, work, or the amount of heat.
Heat transfer is a
process function (or path function), as opposed to
functions of state; therefore, the amount of heat transferred in a
thermodynamic process that changes the
state of a
system
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its open system (systems theory), environment, is described by its boundaries, str ...
depends on how that process occurs, not only the net difference between the initial and final states of the process.
Thermodynamic and
mechanical heat transfer is calculated with the
heat transfer coefficient, the
proportionality between the
heat flux and the thermodynamic driving force for the flow of heat. Heat flux is a quantitative, vectorial representation of heat flow through a surface.
In engineering contexts, the term ''heat'' is taken as synonymous with thermal energy. This usage has its origin in the
historical interpretation of heat as a fluid (''caloric'') that can be transferred by various causes,
[
] and that is also common in the language of laymen and everyday life.
The
transport
Transport (in British English) or transportation (in American English) is the intentional Motion, movement of humans, animals, and cargo, goods from one location to another. Mode of transport, Modes of transport include aviation, air, land tr ...
equations for thermal energy (
Fourier's law
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy ...
), mechanical momentum (
Newton's law for fluids), and mass transfer (
Fick's laws of diffusion) are similar,
[
] and analogies among these three transport processes have been developed to facilitate the prediction of conversion from any one to the others.
[
Thermal engineering concerns the generation, use, conversion, storage, and exchange of heat transfer. As such, heat transfer is involved in almost every sector of the economy. Heat transfer is classified into various mechanisms, such as ]thermal conduction
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy ...
, thermal convection, thermal radiation, and transfer of energy by phase changes.
Mechanisms
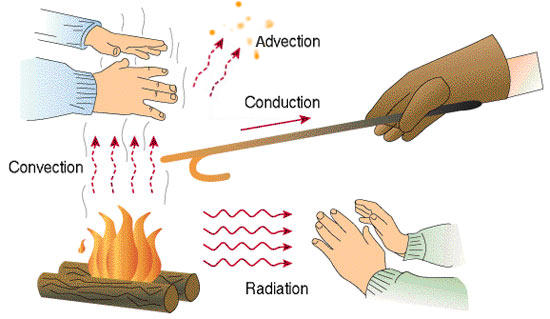 The fundamental modes of heat transfer are:
; Advection
: Advection is the transport mechanism of a
The fundamental modes of heat transfer are:
; Advection
: Advection is the transport mechanism of a fluid
In physics, a fluid is a liquid, gas, or other material that may continuously motion, move and Deformation (physics), deform (''flow'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are M ...
from one location to another, and is dependent on motion
In physics, motion is when an object changes its position with respect to a reference point in a given time. Motion is mathematically described in terms of displacement, distance, velocity, acceleration, speed, and frame of reference to an o ...
and momentum of that fluid.
; Conduction or diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
: The transfer of energy between objects that are in physical contact. Thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
is the property of a material to conduct heat and is evaluated primarily in terms of Fourier's law
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy ...
for heat conduction.
;Convection
Convection is single or Multiphase flow, multiphase fluid flow that occurs Spontaneous process, spontaneously through the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoy ...
: The transfer of energy between an object and its environment, due to fluid motion. The average temperature is a reference for evaluating properties related to convective heat transfer.
;Radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
* ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infr ...
: The transfer of energy by the emission of electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
.
Advection
By transferring matter, energy—including thermal energy—is moved by the physical transfer of a hot or cold object from one place to another. This can be as simple as placing hot water in a bottle and heating a bed, or the movement of an iceberg in changing ocean currents. A practical example is thermal hydraulics. This can be described by the formula:
where
* is heat flux (W/m2),
* is density (kg/m3),
* is heat capacity at constant pressure (J/kg·K),
* is the difference in temperature (K),
* is velocity (m/s).
Conduction
On a microscopic scale, heat conduction occurs as hot, rapidly moving or vibrating atoms and molecules interact with neighboring atoms and molecules, transferring some of their energy (heat) to these neighboring particles. In other words, heat is transferred by conduction when adjacent atoms vibrate against one another, or as electrons move from one atom to another. Conduction is the most significant means of heat transfer within a solid or between solid objects in thermal contact
In heat transfer and thermodynamics, a thermodynamic system
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics.
Thermodynamic systems can be passive ...
. Fluids—especially gases—are less conductive. Thermal contact conductance is the study of heat conduction between solid bodies in contact.Fourier's law
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy ...
). In steady state conduction, the amount of heat entering a section is equal to amount of heat coming out, since the temperature change (a measure of heat energy) is zero.
Convection
The flow of fluid may be forced by external processes, or sometimes (in gravitational fields) by buoyancy forces caused when thermal energy expands the fluid (for example in a fire plume), thus influencing its own transfer. The latter process is often called "natural convection". All convective processes also move heat partly by diffusion, as well. Another form of convection is forced convection. In this case, the fluid is forced to flow by using a pump, fan, or other mechanical means.
Convective heat transfer, or simply, convection, is the transfer of heat from one place to another by the movement of fluids, a process that is essentially the transfer of heat via mass transfer. The bulk motion of fluid enhances heat transfer in many physical situations, such as between a solid surface and the fluid. Convection is usually the dominant form of heat transfer in liquids and gases. Although sometimes discussed as a third method of heat transfer, convection is usually used to describe the combined effects of heat conduction within the fluid (diffusion) and heat transference by bulk fluid flow streaming. The process of transport by fluid streaming is known as advection, but pure advection is a term that is generally associated only with mass transport in fluids, such as advection of pebbles in a river. In the case of heat transfer in fluids, where transport by advection in a fluid is always also accompanied by transport via heat diffusion (also known as heat conduction) the process of heat convection is understood to refer to the sum of heat transport by advection and diffusion/conduction.
Free, or natural, convection occurs when bulk fluid motions (streams and currents) are caused by buoyancy forces that result from density variations due to variations of temperature in the fluid. ''Forced'' convection is a term used when the streams and currents in the fluid are induced by external means—such as fans, stirrers, and pumps—creating an artificially induced convection current.
Convection-cooling
Convective cooling is sometimes described as Newton's law of cooling
In the study of heat transfer, Newton's law of cooling is a physical law which states that the rate of heat loss of a body is directly proportional to the difference in the temperatures between the body and its environment. The law is frequentl ...
:
However, by definition, the validity of Newton's law of cooling requires that the rate of heat loss from convection be a linear function of ("proportional to") the temperature difference that drives heat transfer, and in convective cooling this is sometimes not the case. In general, convection is not linearly dependent on temperature gradients, and in some cases is strongly nonlinear. In these cases, Newton's law does not apply.
Convection vs. conduction
In a body of fluid that is heated from underneath its container, conduction, and convection can be considered to compete for dominance. If heat conduction is too great, fluid moving down by convection is heated by conduction so fast that its downward movement will be stopped due to its buoyancy
Buoyancy (), or upthrust, is the force exerted by a fluid opposing the weight of a partially or fully immersed object (which may be also be a parcel of fluid). In a column of fluid, pressure increases with depth as a result of the weight of t ...
, while fluid moving up by convection is cooled by conduction so fast that its driving buoyancy will diminish. On the other hand, if heat conduction is very low, a large temperature gradient may be formed and convection might be very strong.
The Rayleigh number () is the product of the Grashof () and Prandtl () numbers. It is a measure that determines the relative strength of conduction and convection.
where
* ''g'' is the acceleration due to gravity,
* ''ρ'' is the density with being the density difference between the lower and upper ends,
* ''μ'' is the dynamic viscosity,
* ''α'' is the Thermal diffusivity
In thermodynamics, thermal diffusivity is the thermal conductivity divided by density and specific heat capacity at constant pressure. It is a measure of the rate of heat transfer inside a material and has SI, SI units of m2/s. It is an intensive ...
,
* ''β'' is the volume thermal expansivity (sometimes denoted ''α'' elsewhere),
* ''T'' is the temperature,
* ''ν'' is the kinematic viscosity, and
* ''L'' is characteristic length.
The Rayleigh number can be understood as the ratio between the rate of heat transfer by convection to the rate of heat transfer by conduction; or, equivalently, the ratio between the corresponding timescales (i.e. conduction timescale divided by convection timescale), up to a numerical factor. This can be seen as follows, where all calculations are up to numerical factors depending on the geometry of the system.
The buoyancy force driving the convection is roughly , so the corresponding pressure is roughly . In steady state, this is canceled by the shear stress
Shear stress (often denoted by , Greek alphabet, Greek: tau) is the component of stress (physics), stress coplanar with a material cross section. It arises from the shear force, the component of force vector parallel to the material cross secti ...
due to viscosity, and therefore roughly equals , where ''V'' is the typical fluid velocity due to convection and the order of its timescale. The conduction timescale, on the other hand, is of the order of .
Convection occurs when the Rayleigh number is above 1,000–2,000.
Radiation
 Radiative heat transfer is the transfer of energy via thermal radiation, i.e.,
Radiative heat transfer is the transfer of energy via thermal radiation, i.e., electromagnetic waves
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength, ran ...
.solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
or fluid
In physics, a fluid is a liquid, gas, or other material that may continuously motion, move and Deformation (physics), deform (''flow'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are M ...
or gas). Thermal radiation is emitted by all objects at temperatures above absolute zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 ° ...
, due to random movements of atoms and molecules in matter. Since these atoms and molecules are composed of charged particles (proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s), their movement results in the emission of electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
which carries away energy. Radiation is typically only important in engineering applications for very hot objects, or for objects with a large temperature difference.
When the objects and distances separating them are large in size and compared to the wavelength of thermal radiation, the rate of transfer of radiant energy
In physics, and in particular as measured by radiometry, radiant energy is the energy of electromagnetic radiation, electromagnetic and gravitational radiation. As energy, its SI unit is the joule (J). The quantity of radiant energy may be calcul ...
is best described by the Stefan-Boltzmann equation. For an object in vacuum, the equation is:
For radiative transfer between two objects, the equation is as follows:
where
* is the heat flux,
* is the emissivity (unity for a black body),
* is the Stefan–Boltzmann constant,
* is the view factor between two surfaces a and b, and
* and are the absolute temperatures (in kelvins or degrees Rankine) for the two objects.
The blackbody limit established by the Stefan-Boltzmann equation can be exceeded when the objects exchanging thermal radiation or the distances separating them are comparable in scale or smaller than the dominant thermal wavelength. The study of these cases is called near-field radiative heat transfer.
Radiation from the sun, or solar radiation, can be harvested for heat and power. Unlike conductive and convective forms of heat transfer, thermal radiation – arriving within a narrow-angle i.e. coming from a source much smaller than its distance – can be concentrated in a small spot by using reflecting mirrors, which is exploited in concentrating solar power generation or a burning glass. For example, the sunlight reflected from mirrors heats the PS10 solar power tower and during the day it can heat water to .
The reachable temperature at the target is limited by the temperature of the hot source of radiation. (T4-law lets the reverse flow of radiation back to the source rise.) The (on its surface) somewhat 4000 K hot sun allows to reach coarsely 3000 K (or 3000 °C, which is about 3273 K) at a small probe in the focus spot of a big concave, concentrating mirror of the Mont-Louis Solar Furnace in France.
Phase transition
Phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
or phase change, takes place in a thermodynamic system
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics.
Thermodynamic systems can be passive and active according to internal processes. According to inter ...
from one phase or state of matter
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and Plasma (physics), plasma.
Different states are distinguished by the ways the ...
to another one by heat transfer. Phase change examples are the melting of ice or the boiling of water.
The Mason equation explains the growth of a water droplet based on the effects of heat transport on evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
and condensation.
Phase transitions involve the four fundamental states of matter:
* Solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
– Deposition, freezing, and solid-to-solid transformation.
* Liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
– Condensation and melting / fusion.
* Gas – Boiling / evaporation, recombination/ deionization, and sublimation.
* Plasma – Ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged at ...
.
Boiling
The boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid evaporates resulting in an abrupt change in vapor volume.
In a closed system, ''saturation temperature'' and ''boiling point'' mean the same thing. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. The liquid can be said to be saturated with thermal energy. Any addition of thermal energy results in a phase transition.
At standard atmospheric pressure and low temperatures, no boiling occurs and the heat transfer rate is controlled by the usual single-phase mechanisms. As the surface temperature is increased, local boiling occurs and vapor bubbles nucleate, grow into the surrounding cooler fluid, and collapse. This is ''sub-cooled nucleate boiling'', and is a very efficient heat transfer mechanism. At high bubble generation rates, the bubbles begin to interfere and the heat flux no longer increases rapidly with surface temperature (this is the departure from nucleate boiling, or DNB).
At similar standard atmospheric pressure and high temperatures, the hydrodynamically quieter regime of film boiling is reached. Heat fluxes across the stable vapor layers are low but rise slowly with temperature. Any contact between the fluid and the surface that may be seen probably leads to the extremely rapid nucleation of a fresh vapor layer ("spontaneous nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically def ...
"). At higher temperatures still, a maximum in the heat flux is reached (the critical heat flux, or CHF).
The Leidenfrost Effect demonstrates how nucleate boiling slows heat transfer due to gas bubbles on the heater's surface. As mentioned, gas-phase thermal conductivity is much lower than liquid-phase thermal conductivity, so the outcome is a kind of "gas thermal barrier".
Condensation
Condensation occurs when a vapor is cooled and changes its phase to a liquid. During condensation, the latent heat of vaporization must be released. The amount of heat is the same as that absorbed during vaporization at the same fluid pressure.
There are several types of condensation:
* Homogeneous condensation, as during the formation of fog.
* Condensation in direct contact with subcooled liquid.
* Condensation on direct contact with a cooling wall of a heat exchanger: This is the most common mode used in industry: Dropwise condensation is difficult to sustain reliably; therefore, industrial equipment is normally designed to operate in filmwise condensation mode.
Melting
 Melting is a thermal process that results in the phase transition of a substance from a
Melting is a thermal process that results in the phase transition of a substance from a solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
to a liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
. The internal energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accoun ...
of a substance is increased, typically through heat or pressure, resulting in a rise of its temperature to the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
, at which the ordering of ionic or molecular entities in the solid breaks down to a less ordered state and the solid liquefies. Molten substances generally have reduced viscosity with elevated temperature; an exception to this maxim is the element sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, whose viscosity increases to a point due to polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
and then decreases with higher temperatures in its molten state.
Modeling approaches
Heat transfer can be modeled in various ways.
Heat equation
The heat equation is an important partial differential equation
In mathematics, a partial differential equation (PDE) is an equation which involves a multivariable function and one or more of its partial derivatives.
The function is often thought of as an "unknown" that solves the equation, similar to ho ...
that describes the distribution of heat (or temperature variation) in a given region over time. In some cases, exact solutions of the equation are available;
Lumped system analysis
Lumped system analysis often reduces the complexity of the equations to one first-order linear differential equation, in which case heating and cooling are described by a simple exponential solution, often referred to as Newton's law of cooling
In the study of heat transfer, Newton's law of cooling is a physical law which states that the rate of heat loss of a body is directly proportional to the difference in the temperatures between the body and its environment. The law is frequentl ...
.
System analysis by the lumped capacitance model is a common approximation in transient conduction that may be used whenever heat conduction within an object is much faster than heat conduction across the boundary of the object. This is a method of approximation that reduces one aspect of the transient conduction system—that within the object—to an equivalent steady-state system. That is, the method assumes that the temperature within the object is completely uniform, although its value may change over time.
In this method, the ratio of the conductive heat resistance within the object to the convective heat transfer resistance across the object's boundary, known as the ''Biot number
The Biot number (Bi) is a dimensionless quantity used in heat transfer calculations, named for the eighteenth-century French physicist Jean-Baptiste Biot (1774–1862). The Biot number is the ratio of the thermal resistance for conduction inside ...
'', is calculated. For small Biot numbers, the approximation of ''spatially uniform temperature within the object'' can be used: it can be presumed that heat transferred into the object has time to uniformly distribute itself, due to the lower resistance to doing so, as compared with the resistance to heat entering the object.
Climate models
Climate models study the radiant heat transfer by using quantitative methods to simulate the interactions of the atmosphere, oceans, land surface, and ice.
Engineering
 Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances. Heat transfer methods are used in numerous disciplines, such as automotive engineering, thermal management of electronic devices and systems, climate control, insulation, materials processing,
Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances. Heat transfer methods are used in numerous disciplines, such as automotive engineering, thermal management of electronic devices and systems, climate control, insulation, materials processing, chemical engineering
Chemical engineering is an engineering field which deals with the study of the operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials ...
and power station
A power station, also referred to as a power plant and sometimes generating station or generating plant, is an industrial facility for the electricity generation, generation of electric power. Power stations are generally connected to an electr ...
engineering.
Insulation, radiance and resistance
Thermal insulators are materials specifically designed to reduce the flow of heat by limiting conduction, convection, or both. Thermal resistance is a heat property and the measurement by which an object or material resists to heat flow (heat per time unit or thermal resistance) to temperature difference.
Radiance, or spectral radiance, is a measure of the quantity of radiation that passes through or is emitted. Radiant barriers are materials that reflect radiation, and therefore reduce the flow of heat from radiation sources. Good insulators are not necessarily good radiant barriers, and vice versa. Metal, for instance, is an excellent reflector and a poor insulator.
The effectiveness of a radiant barrier is indicated by its reflectivity, which is the fraction of radiation reflected. A material with a high reflectivity (at a given wavelength) has a low emissivity (at that same wavelength), and vice versa. At any specific wavelength, reflectivity=1 - emissivity. An ideal radiant barrier would have a reflectivity of 1, and would therefore reflect 100 percent of incoming radiation. Vacuum flasks, or Dewars, are silvered to approach this ideal. In the vacuum of space, satellites use multi-layer insulation, which consists of many layers of aluminized (shiny) Mylar to greatly reduce radiation heat transfer and control satellite temperature.
Devices
A heat engine is a system that performs the conversion of a flow of thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
(heat) to mechanical energy to perform mechanical work.
A thermocouple is a temperature-measuring device and a widely used type of temperature sensor for measurement and control, and can also be used to convert heat into electric power.
A thermoelectric cooler is a solid-state electronic device that pumps (transfers) heat from one side of the device to the other when an electric current is passed through it. It is based on the Peltier effect.
A thermal diode or thermal rectifier is a device that causes heat to flow preferentially in one direction.
Heat exchangers
A heat exchanger is used for more efficient heat transfer or to dissipate heat. Heat exchangers are widely used in refrigeration
Refrigeration is any of various types of cooling of a space, substance, or system to lower and/or maintain its temperature below the ambient one (while the removed heat is ejected to a place of higher temperature).IIR International Dictionary of ...
, air conditioning
Air conditioning, often abbreviated as A/C (US) or air con (UK), is the process of removing heat from an enclosed space to achieve a more comfortable interior temperature, and in some cases, also controlling the humidity of internal air. Air c ...
, space heating, power generation, and chemical processing. One common example of a heat exchanger is a car's radiator, in which the hot coolant fluid is cooled by the flow of air over the radiator's surface.
Common types of heat exchanger flows include parallel flow, counter flow, and cross flow. In parallel flow, both fluids move in the same direction while transferring heat; in counter flow, the fluids move in opposite directions; and in cross flow, the fluids move at right angle
In geometry and trigonometry, a right angle is an angle of exactly 90 Degree (angle), degrees or radians corresponding to a quarter turn (geometry), turn. If a Line (mathematics)#Ray, ray is placed so that its endpoint is on a line and the ad ...
s to each other. Common types of heat exchangers include shell and tube, double pipe, extruded finned pipe, spiral fin pipe, u-tube, and stacked plate. Each type has certain advantages and disadvantages over other types.
A heat sink
A heat sink (also commonly spelled heatsink) is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant, where it is thermal management (electronics), ...
is a component that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems or the radiator in a car. A heat pipe is another heat-transfer device that combines thermal conductivity and phase transition to efficiently transfer heat between two solid interfaces.
Applications
Architecture
Efficient energy use
Efficient energy use, or energy efficiency, is the process of reducing the amount of energy required to provide products and services. There are many technologies and methods available that are more energy efficient than conventional systems. For ...
is the goal to reduce the amount of energy required in heating or cooling. In architecture, condensation and air currents can cause cosmetic or structural damage. An energy audit
Energy () is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat and light. Energy is a conserved quantity—the law of conservation of ener ...
can help to assess the implementation of recommended corrective procedures. For instance, insulation improvements, air sealing of structural leaks, or the addition of energy-efficient windows and doors.
* Smart meter is a device that records electric energy consumption in intervals.
* Thermal transmittance is the rate of transfer of heat through a structure divided by the difference in temperature across the structure. It is expressed in watts per square meter per kelvin, or W/(m2K). Well-insulated parts of a building have a low thermal transmittance, whereas poorly-insulated parts of a building have a high thermal transmittance.
* Thermostat
A thermostat is a regulating device component which senses the temperature of a physical system and performs actions so that the system's temperature is maintained near a desired setpoint.
Thermostats are used in any device or system tha ...
is a device to monitor and control temperature.
Climate engineering
 Climate engineering consists of carbon dioxide removal and solar radiation management. Since the amount of
Climate engineering consists of carbon dioxide removal and solar radiation management. Since the amount of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
determines the radiative balance of Earth's atmosphere, carbon dioxide removal techniques can be applied to reduce the radiative forcing. Solar radiation management is the attempt to absorb less solar radiation to offset the effects of greenhouse gases.
An alternative method is passive daytime radiative cooling
Passive daytime radiative cooling (PDRC) (also passive radiative cooling, daytime passive radiative cooling, radiative sky cooling, photonic radiative cooling, and terrestrial radiative cooling) is the use of unpowered, reflective/Emissivity, ther ...
, which enhances terrestrial heat flow to outer space through the infrared window (8–13 μm).
Greenhouse effect
 The
The greenhouse effect
The greenhouse effect occurs when greenhouse gases in a planet's atmosphere insulate the planet from losing heat to space, raising its surface temperature. Surface heating can happen from an internal heat source (as in the case of Jupiter) or ...
is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases and clouds, and is re-radiated in all directions, resulting in a reduction in the amount of thermal radiation reaching space relative to what would reach space in the absence of absorbing materials. This reduction in outgoing radiation leads to a rise in the temperature of the surface and troposphere until the rate of outgoing radiation again equals the rate at which heat arrives from the Sun.
Heat transfer in the human body
The principles of heat transfer in engineering systems can be applied to the human body to determine how the body transfers heat. Heat is produced in the body by the continuous metabolism of nutrients which provides energy for the systems of the body. The human body must maintain a consistent internal temperature to maintain healthy bodily functions. Therefore, excess heat must be dissipated from the body to keep it from overheating. When a person engages in elevated levels of physical activity, the body requires additional fuel which increases the metabolic rate and the rate of heat production. The body must then use additional methods to remove the additional heat produced to keep the internal temperature at a healthy level.
Heat transfer by convection is driven by the movement of fluids over the surface of the body. This convective fluid can be either a liquid or a gas. For heat transfer from the outer surface of the body, the convection mechanism is dependent on the surface area of the body, the velocity of the air, and the temperature gradient between the surface of the skin and the ambient air.[Cengel, Yunus A. and Ghajar, Afshin J. "Heat and Mass Transfer: Fundamentals and Applications", McGraw-Hill, 4th Edition, 2010.] The normal temperature of the body is approximately 37 °C. Heat transfer occurs more readily when the temperature of the surroundings is significantly less than the normal body temperature. This concept explains why a person feels cold when not enough covering is worn when exposed to a cold environment. Clothing can be considered an insulator which provides thermal resistance to heat flow over the covered portion of the body. This thermal resistance causes the temperature on the surface of the clothing to be less than the temperature on the surface of the skin. This smaller temperature gradient between the surface temperature and the ambient temperature will cause a lower rate of heat transfer than if the skin were not covered.
To ensure that one portion of the body is not significantly hotter than another portion, heat must be distributed evenly through the bodily tissues. Blood flowing through blood vessels acts as a convective fluid and helps to prevent any buildup of excess heat inside the tissues of the body. This flow of blood through the vessels can be modeled as pipe flow in an engineering system. The heat carried by the blood is determined by the temperature of the surrounding tissue, the diameter of the blood vessel, the thickness of the fluid, the velocity of the flow, and the heat transfer coefficient of the blood. The velocity, blood vessel diameter, and fluid thickness can all be related to the Reynolds Number
In fluid dynamics, the Reynolds number () is a dimensionless quantity that helps predict fluid flow patterns in different situations by measuring the ratio between Inertia, inertial and viscous forces. At low Reynolds numbers, flows tend to ...
, a dimensionless number used in fluid mechanics to characterize the flow of fluids.
Latent heat loss, also known as evaporative heat loss, accounts for a large fraction of heat loss from the body. When the core temperature of the body increases, the body triggers sweat glands in the skin to bring additional moisture to the surface of the skin. The liquid is then transformed into vapor which removes heat from the surface of the body. The rate of evaporation heat loss is directly related to the vapor pressure at the skin surface and the amount of moisture present on the skin.
Cooling techniques
Evaporative cooling
 Evaporative cooling happens when water vapor is added to the surrounding air. The energy needed to evaporate the water is taken from the air in the form of sensible heat and converted into latent heat, while the air remains at a constant enthalpy. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in the air occurs; thus, there is no cooling effect.
Evaporative cooling happens when water vapor is added to the surrounding air. The energy needed to evaporate the water is taken from the air in the form of sensible heat and converted into latent heat, while the air remains at a constant enthalpy. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in the air occurs; thus, there is no cooling effect.
Laser cooling
In quantum physics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
, laser cooling
Laser cooling includes several techniques where atoms, molecules, and small mechanical systems are cooled with laser light. The directed energy of lasers is often associated with heating materials, e.g. laser cutting, so it can be counterintuit ...
is used to achieve temperatures of near absolute zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 ° ...
(−273.15 °C, −459.67 °F) of atomic and molecular samples to observe unique quantum effects that can only occur at this heat level.
* Doppler cooling is the most common method of laser cooling.
* Sympathetic cooling is a process in which particles of one type cool particles of another type. Typically, atomic ions that can be directly laser-cooled are used to cool nearby ions or atoms. This technique allows the cooling of ions and atoms that cannot be laser-cooled directly.
Magnetic cooling
Magnetic evaporative cooling
Evaporative cooling is an atomic physics technique to achieve high phase space densities which optical cooling techniques alone typically can not reach.
Atoms trapped in optical or magnetic traps can be evaporatively cooled via two primary mecha ...
is a process for lowering the temperature of a group of atoms, after pre-cooled by methods such as laser cooling. Magnetic refrigeration cools below 0.3K, by making use of the magnetocaloric effect.
Radiative cooling
Radiative cooling
In the study of heat transfer, radiative cooling is the process by which a body loses heat by thermal radiation. As Planck's law describes, every physical body spontaneously and continuously emits electromagnetic radiation.
Radiative cooling has b ...
is the process by which a body loses heat by radiation. Outgoing energy is an important effect in the Earth's energy budget. In the case of the Earth-atmosphere system, it refers to the process by which long-wave (infrared) radiation is emitted to balance the absorption of short-wave (visible) energy from the Sun. The thermosphere (top of atmosphere) cools to space primarily by infrared energy radiated by carbon dioxide () at 15 μm and by nitric oxide (NO) at 5.3 μm. Convective transport of heat and evaporative transport of latent heat both remove heat from the surface and redistribute it in the atmosphere.
Thermal energy storage
Thermal energy storage includes technologies for collecting and storing energy for later use. It may be employed to balance energy demand between day and nighttime. The thermal reservoir may be maintained at a temperature above or below that of the ambient environment. Applications include space heating, domestic or process hot water systems, or generating electricity.
History
Newton's law of cooling
In 1701, Isaac Newton
Sir Isaac Newton () was an English polymath active as a mathematician, physicist, astronomer, alchemist, theologian, and author. Newton was a key figure in the Scientific Revolution and the Age of Enlightenment, Enlightenment that followed ...
anonymously published an article in '' Philosophical Transactions'' noting (in modern terms) that the rate of temperature change of a body is proportional to the difference in temperatures (, "degrees of heat") between the body and its surroundings. The phrase "temperature change" was later replaced with "heat loss", and the relationship was named Newton's law of cooling. In general, the law is valid only if the temperature difference is small and the heat transfer mechanism remains the same.
Thermal conduction
In heat conduction, the law is valid only if the thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
of the warmer body is independent of temperature. The thermal conductivity of most materials is only weakly dependent on temperature, so in general the law holds true.
Thermal convection
In convective heat transfer, the law is valid for forced air or pumped fluid cooling, where the properties of the fluid do not vary strongly with temperature, but it is only approximately true for buoyancy-driven convection, where the velocity of the flow increases with temperature difference.
Thermal radiation
In the case of heat transfer by thermal radiation, Newton's law of cooling holds only for very small temperature differences.
Thermal conductivity of different metals
In a 1780 letter to Benjamin Franklin
Benjamin Franklin (April 17, 1790) was an American polymath: a writer, scientist, inventor, statesman, diplomat, printer, publisher and Political philosophy, political philosopher.#britannica, Encyclopædia Britannica, Wood, 2021 Among the m ...
, Dutch-born British scientist Jan Ingenhousz relates an experiment which enabled him to rank seven different metals according to their thermal conductivities:
Benjamin Thompson's experiments on heat transfer
During the years 1784 – 1798, the British physicist Benjamin Thompson (Count Rumford) lived in Bavaria
Bavaria, officially the Free State of Bavaria, is a States of Germany, state in the southeast of Germany. With an area of , it is the list of German states by area, largest German state by land area, comprising approximately 1/5 of the total l ...
, reorganizing the Bavarian army for the Prince-elector Charles Theodore among other official and charitable duties. The Elector gave Thompson access to the facilities of the Electoral Academy of Sciences in Mannheim
Mannheim (; Palatine German language, Palatine German: or ), officially the University City of Mannheim (), is the List of cities in Baden-Württemberg by population, second-largest city in Baden-Württemberg after Stuttgart, the States of Ger ...
. During his years in Mannheim and later in Munich
Munich is the capital and most populous city of Bavaria, Germany. As of 30 November 2024, its population was 1,604,384, making it the third-largest city in Germany after Berlin and Hamburg. Munich is the largest city in Germany that is no ...
, Thompson made a large number of discoveries and inventions related to heat.
Conductivity experiments
= "New Experiments upon Heat"
=
In 1785 Thompson performed a series of thermal conductivity experiments, which he describes in great detail in the '' Philosophical Transactions'' article "New Experiments upon Heat" from 1786. The fact that good electrical conductors are often also good heat conductors and vice versa must have been well known at the time, for Thompson mentions it in passing. He intended to measure the relative conductivities of mercury, water, moist air, "common air" (dry air at normal atmospheric pressure), dry air of various rarefication, and a " Torricellian vacuum".
For these experiments, Thompson employed a thermometer inside a large, closed glass tube. Under the circumstances described, heat may—unbeknownst to Thompson—have been transferred more by radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
* ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infr ...
than by conduction. These were his results.
After the experiments, Thompson was surprised to observe that a vacuum was a significantly poorer heat conductor than air "which of itself is reckoned among the worst", but only a very small difference between common air and rarefied air. He also noted the great difference between dry air and moist air, and the great benefit this affords.
Temperature vs. sensible heat
Thompson concluded with some comments on the important difference between temperature and sensible heat.
Coining of the term "convection"
In the 1830s, in '' The Bridgewater Treatises'', the term ''convection'' is attested in a scientific sense. In treatise VIII by William Prout, in the book on chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, it says:This motion of heat takes place in three ways, which a common fire-place very well illustrates. If, for instance, we place a thermometer directly before a fire, it soon begins to rise, indicating an increase of temperature. In this case the heat has made its way through the space between the fire and the thermometer, by the process termed ''radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
* ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infr ...
''. If we place a second thermometer in contact with any part of the grate, and away from the direct influence of the fire, we shall find that this thermometer also denotes an increase of temperature; but here the heat must have travelled through the metal of the grate, by what is termed '' conduction''. Lastly, a third thermometer placed in the chimney, away from the direct influence of the fire, will also indicate a considerable increase of temperature; in this case a portion of the air, passing through and near the fire, has become heated, and has ''carried'' up the chimney the temperature acquired from the fire. There is at present no single term in our language employed to denote this third mode of the propagation of heat; but we venture to propose for that purpose, the term ''convection'', n footnote: [Latin''Convectio'', a carrying or conveying">atin">n footnote: [Latin''Convectio'', a carrying or conveyingwhich not only expresses the leading fact, but also accords very well with the two other terms.
Later, in the same treatise VIII, in the book on meteorology, the concept of convection is also applied to "the process by which heat is communicated through water".
See also
* Combined forced and natural convection
* Heat capacity
* Heat transfer enhancement
Heat transfer enhancement is the process of increasing the effectiveness of Heat exchanger, heat exchangers. This can be achieved when the heat transfer power of a given device is increased or when the pressure losses generated by the device are re ...
* Heat transfer physics
* Stefan–Boltzmann law
* Thermal contact conductance
* Thermal energy storage
* Thermal physics
* Thermal resistance
Citations
References
*
*
External links
A Heat Transfer Textbook
- (free download).
Thermal-FluidsPedia
- An online thermal fluids encyclopedia.
- Overview
- a practical example of how heat transfer is used to heat buildings without burning fossil fuels.
Thermal-Fluids Central
{{DEFAULTSORT:Heat Transfer
Chemical engineering
Mechanical engineering
Unit operations
Transport phenomena
 Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of  Heat transfer is the energy exchanged between materials (solid/liquid/gas) as a result of a temperature difference. The
Heat transfer is the energy exchanged between materials (solid/liquid/gas) as a result of a temperature difference. The  The fundamental modes of heat transfer are:
; Advection
: Advection is the transport mechanism of a
The fundamental modes of heat transfer are:
; Advection
: Advection is the transport mechanism of a  Radiative heat transfer is the transfer of energy via thermal radiation, i.e.,
Radiative heat transfer is the transfer of energy via thermal radiation, i.e.,  Melting is a thermal process that results in the phase transition of a substance from a
Melting is a thermal process that results in the phase transition of a substance from a  Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances. Heat transfer methods are used in numerous disciplines, such as automotive engineering, thermal management of electronic devices and systems, climate control, insulation, materials processing,
Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances. Heat transfer methods are used in numerous disciplines, such as automotive engineering, thermal management of electronic devices and systems, climate control, insulation, materials processing,  Climate engineering consists of carbon dioxide removal and solar radiation management. Since the amount of
Climate engineering consists of carbon dioxide removal and solar radiation management. Since the amount of  Evaporative cooling happens when water vapor is added to the surrounding air. The energy needed to evaporate the water is taken from the air in the form of sensible heat and converted into latent heat, while the air remains at a constant enthalpy. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in the air occurs; thus, there is no cooling effect.
Evaporative cooling happens when water vapor is added to the surrounding air. The energy needed to evaporate the water is taken from the air in the form of sensible heat and converted into latent heat, while the air remains at a constant enthalpy. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in the air occurs; thus, there is no cooling effect.