Gas electron diffraction on:
[Wikipedia]
[Google]
[Amazon]
Gas electron diffraction (GED) is one of the applications of


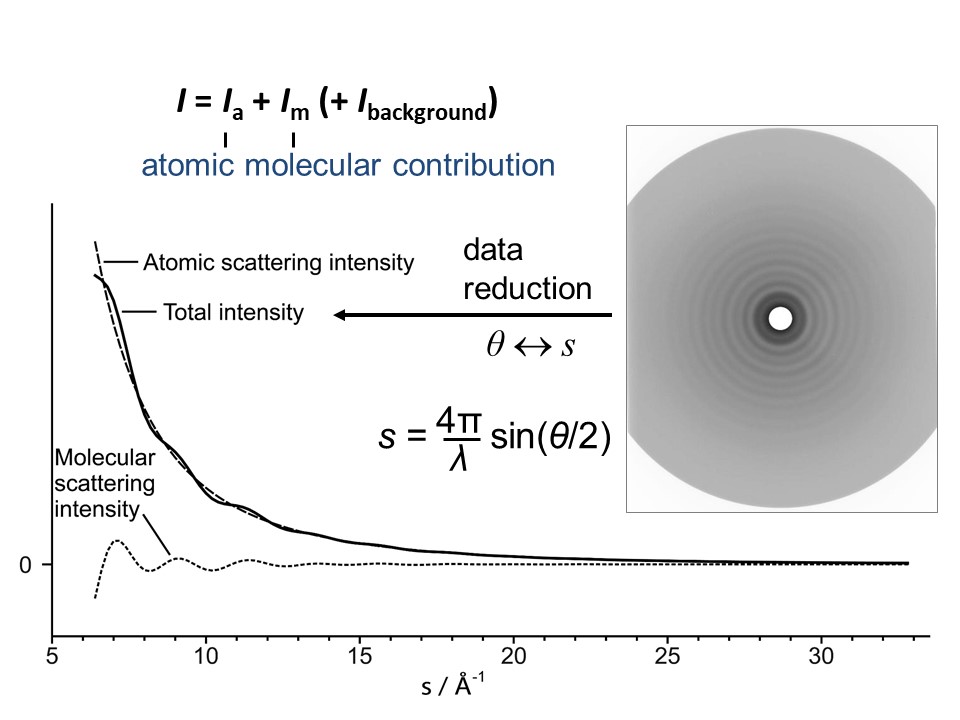 Figure 1 shows a drawing and a photograph of an electron diffraction apparatus. Scheme 1 shows the schematic procedure of an electron diffraction experiment. A fast
Figure 1 shows a drawing and a photograph of an electron diffraction apparatus. Scheme 1 shows the schematic procedure of an electron diffraction experiment. A fast 
 The scattering pattern consists of diffuse concentric rings (see Figure 2). The steep decent of intensity can be compensated for by passing the electrons through a fast rotation sector (Figure 3). This is cut in a way, that electrons with small scattering angles are more shadowed than those at wider scattering angles. The detector can be a
The scattering pattern consists of diffuse concentric rings (see Figure 2). The steep decent of intensity can be compensated for by passing the electrons through a fast rotation sector (Figure 3). This is cut in a way, that electrons with small scattering angles are more shadowed than those at wider scattering angles. The detector can be a
UNEX
for refining a suitable model for the compound and to yield precise structural information in terms of bond lengths, angles and torsional angles.

 GED can be described by scattering theory. The outcome if applied to gases with randomly oriented molecules is provided here in short:
Scattering occurs at each individual atom (), but also at pairs (also called molecular scattering) (), or triples (), of atoms.
is the scattering variable or change of electron
GED can be described by scattering theory. The outcome if applied to gases with randomly oriented molecules is provided here in short:
Scattering occurs at each individual atom (), but also at pairs (also called molecular scattering) (), or triples (), of atoms.
is the scattering variable or change of electron
 Figure 5 shows two typical examples of results. The molecular scattering intensity curves are used to refine a structural model by means of a
Figure 5 shows two typical examples of results. The molecular scattering intensity curves are used to refine a structural model by means of a
program
This yield precise structural information. The Fourier transformation of the molecular scattering intensity curves gives the radial distribution curves (RDC). These represent the probability to find a certain distance between two nuclei of a molecule. The curves below the RDC represent the diffrerence between the experiment and the model, i.e. the quality of fit. The very simple example in Figure 5 shows the results for evaporated white
Norway
electron diffraction
Electron diffraction is a generic term for phenomena associated with changes in the direction of electron beams due to elastic interactions with atoms. It occurs due to elastic scattering, when there is no change in the energy of the electrons. ...
techniques. The target of this method is the determination of the structure of gaseous molecules, i.e., the geometrical arrangement of the atoms from which a molecule is built up. GED is one of two experimental methods (besides microwave spectroscopy) to determine the structure of free molecules, undistorted by intermolecular forces, which are omnipresent in the solid and liquid state. The determination of accurate molecular structures by GED studies is fundamental for an understanding of structural chemistry.
Introduction
Diffraction occurs because thewavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
of electrons accelerated by a potential of a few thousand volts is of the same order of magnitude as internuclear distances in molecules. The principle is the same as that of other electron diffraction methods such as LEED
Leadership in Energy and Environmental Design (LEED) is a Green building certification systems, green building certification program used worldwide. Developed by the non-profit U.S. Green Building Council (USGBC), it includes a set of rating ...
and RHEED, but the obtainable diffraction pattern is considerably weaker than those of LEED and RHEED because the density of the target is about one thousand times smaller. Since the orientation of the target molecules relative to the electron beams is random, the internuclear distance information obtained is one-dimensional. Thus only relatively simple molecules can be completely structurally characterized by electron diffraction in the gas phase. It is possible to combine information obtained from other sources, such as rotational spectra, NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
or high-quality quantum-mechanical calculations with electron diffraction data, if the latter are not sufficient to determine the molecule's structure completely.
The total scattering intensity in GED is given as a function of the momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
transfer, which is defined as the difference between the wave vector
In physics, a wave vector (or wavevector) is a vector used in describing a wave, with a typical unit being cycle per metre. It has a magnitude and direction. Its magnitude is the wavenumber of the wave (inversely proportional to the wavelength) ...
of the incident electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
beam and that of the scattered electron beam and has the reciprocal dimension of length
Length is a measure of distance. In the International System of Quantities, length is a quantity with Dimension (physical quantity), dimension distance. In most systems of measurement a Base unit (measurement), base unit for length is chosen, ...
. The total scattering intensity is composed of two parts: the atomic scattering intensity and the molecular scattering intensity. The former decreases monotonically and contains no information about the molecular structure. The latter has sinusoidal
A sine wave, sinusoidal wave, or sinusoid (symbol: ∿) is a periodic wave whose waveform (shape) is the trigonometric sine function. In mechanics, as a linear motion over time, this is '' simple harmonic motion''; as rotation, it correspond ...
modulations as a result of the interference
Interference is the act of interfering, invading, or poaching. Interference may also refer to:
Communications
* Interference (communication), anything which alters, modifies, or disrupts a message
* Adjacent-channel interference, caused by extra ...
of the scattering spherical waves generated by the scattering from the atoms included in the target molecule. The interferences reflect the distributions of the atoms composing the molecules, so the molecular structure is determined from this part.

Experiment

 Figure 1 shows a drawing and a photograph of an electron diffraction apparatus. Scheme 1 shows the schematic procedure of an electron diffraction experiment. A fast
Figure 1 shows a drawing and a photograph of an electron diffraction apparatus. Scheme 1 shows the schematic procedure of an electron diffraction experiment. A fast electron beam
Since the mid-20th century, electron-beam technology has provided the basis for a variety of novel and specialized applications in semiconductor manufacturing, microelectromechanical systems, nanoelectromechanical systems, and microscopy.
Mechani ...
is generated in an electron gun, enters a diffraction chamber typically at a vacuum of 10−7 mbar. The electron beam hits a perpendicular stream of a gaseous sample effusing from a nozzle of a small diameter (typically 0.2 mm). At this point, the electrons are scattered. Most of the sample is immediately condensed and frozen onto the surface of a cold trap held at -196 °C (liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
). The scattered electrons are detected on the surface of a suitable detector in a well-defined distance to the point of scattering.

 The scattering pattern consists of diffuse concentric rings (see Figure 2). The steep decent of intensity can be compensated for by passing the electrons through a fast rotation sector (Figure 3). This is cut in a way, that electrons with small scattering angles are more shadowed than those at wider scattering angles. The detector can be a
The scattering pattern consists of diffuse concentric rings (see Figure 2). The steep decent of intensity can be compensated for by passing the electrons through a fast rotation sector (Figure 3). This is cut in a way, that electrons with small scattering angles are more shadowed than those at wider scattering angles. The detector can be a photographic plate
Photographic plates preceded film as the primary medium for capturing images in photography. These plates, made of metal or glass and coated with a light-sensitive emulsion, were integral to early photographic processes such as heliography, d ...
, an electron imaging plate (usual technique today) or other position sensitive devices such as hybrid pixel detectors (future technique).
The intensities generated from reading out the plates or processing intensity data from other detectors are then corrected for the sector effect. They are initially a function of distance between primary beam position and intensity, and then converted into a function of scattering angle. The so-called atomic intensity and the experimental background are subtracted to give the final experimental molecular scattering intensities as a function of ''s'' (the change of momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
).
These data are then processed by suitable fitting software likUNEX
for refining a suitable model for the compound and to yield precise structural information in terms of bond lengths, angles and torsional angles.
Theory

 GED can be described by scattering theory. The outcome if applied to gases with randomly oriented molecules is provided here in short:
Scattering occurs at each individual atom (), but also at pairs (also called molecular scattering) (), or triples (), of atoms.
is the scattering variable or change of electron
GED can be described by scattering theory. The outcome if applied to gases with randomly oriented molecules is provided here in short:
Scattering occurs at each individual atom (), but also at pairs (also called molecular scattering) (), or triples (), of atoms.
is the scattering variable or change of electron momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
, and its absolute value is defined as
:
with being the electron wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
defined above, and being the scattering angle.
The above-mentioned contributions of scattering add up to the total scattering
:
where is the experimental background intensity, which is needed to describe the experiment completely.
The contribution of individual atom scattering is called atomic scattering and easy to calculate:
:
with , being the distance between the point of scattering and the detector, being the intensity of the primary electron beam, and being the scattering amplitude of the ''i''-th atom. In essence, this is a summation over the scattering contributions of all atoms independent of the molecular structure. is the main contribution and easily obtained if the atomic composition of the gas (sum formula) is known.
The most interesting contribution is the molecular scattering, because it contains information about the distance between all pairs of atoms in a molecule (bonded or non-bonded):
:
with being the parameter of main interest: the atomic distance between two atoms, being the mean square amplitude of vibration between the two atoms, the anharmonicity constant (correcting the vibration description for deviations from a purely harmonic model), and is a phase factor, which becomes important if a pair of atoms with very different nuclear charge is involved.
The first part is similar to the atomic scattering, but contains two scattering factors of the involved atoms. Summation is performed over all atom pairs.
is negligible in most cases and not described here in more detail. is mostly determined by fitting and subtracting smooth functions to account for the background contribution.
So it is the molecular scattering intensity that is of interest, and this is obtained by calculation all other contributions and subtracting them from the experimentally measured total scattering function.
Results
 Figure 5 shows two typical examples of results. The molecular scattering intensity curves are used to refine a structural model by means of a
Figure 5 shows two typical examples of results. The molecular scattering intensity curves are used to refine a structural model by means of a least squares
The method of least squares is a mathematical optimization technique that aims to determine the best fit function by minimizing the sum of the squares of the differences between the observed values and the predicted values of the model. The me ...
fittinprogram
This yield precise structural information. The Fourier transformation of the molecular scattering intensity curves gives the radial distribution curves (RDC). These represent the probability to find a certain distance between two nuclei of a molecule. The curves below the RDC represent the diffrerence between the experiment and the model, i.e. the quality of fit. The very simple example in Figure 5 shows the results for evaporated white
phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, P4. It is a perfectly tetrahedral molecule and has thus only one P-P distance. This makes the molecular scattering intensity curve a very simple one; a sine curve which is damped due to molecular vibration. The radial distribution curve (RDC) shows a maximum at 2.1994 Å with a least-squares error of 0.0003 Å, represented as 2.1994(3) Å. The width of the peak represents the molecular vibration and is the result of Fourier transform
In mathematics, the Fourier transform (FT) is an integral transform that takes a function as input then outputs another function that describes the extent to which various frequencies are present in the original function. The output of the tr ...
ation of the damping part. This peak width means that the P-P distance varies by this vibration within a certain range given as a vibrational amplitude ''u'', in this example ''u''T(P‒P) = 0.0560(5) Å.
The slightly more complicated molecule P3As has two different distances P-P and P-As. Because their contributions overlap in the RDC, the peak is broader (also seen in a more rapid damping in the molecular scattering). The determination of these two independent parameters is more difficult and results in less precise parameter values than for P4.
Some selected other examples of important contributions to the structural chemistry of molecules are provided here:
* Structure of diborane B2H6
* Structure of the planar trisilylamine
* Determinations of the structures of gaseous elemental phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
P4 and of the binary P3As
* Determination of the structure of C60 and C70
* Structure of tetranitromethane
* Absence of local C3 symmetry in the simplest phosphonium ylide H2C=PMe3 and in amino-phosphanes like P(NMe2)3 and ylide
An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both ...
s H2C=P(NMe2)3
* Determination of intramolecular London dispersion interaction effects on gas-phase and solid-state structures of diamondoid dimers
Links
* http://molwiki.org/wiki/Main_Page—A free encyclopaedia, mainly focused on molecular structure and dynamics. * The story of gas-phase electron diffraction (GED) iNorway
References
{{DEFAULTSORT:Gas Electron Diffraction Diffraction