gas chromatography–mass spectrometry on:
[Wikipedia]
[Google]
[Amazon]
 Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and
Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and
 These two components, used together, allow a much finer degree of substance identification than either unit used separately. It is not possible to make an accurate identification of a particular molecule by gas chromatography or mass spectrometry alone. The mass spectrometry process normally requires a very pure sample while gas chromatography using a traditional detector (e.g. Flame ionization detector) cannot differentiate between multiple molecules that happen to take the same amount of time to travel through the column (''i.e.'' have the same retention time), which results in two or more molecules that co-elute. Sometimes two different molecules can also have a similar pattern of ionized fragments in a mass spectrometer (mass spectrum). Combining the two processes reduces the possibility of error, as it is extremely unlikely that two different molecules will behave in the same way in both a gas chromatograph and a mass spectrometer. Therefore, when an identifying mass spectrum appears at a characteristic retention time in a GC–MS analysis, it typically increases certainty that the analyte of interest is in the sample.
These two components, used together, allow a much finer degree of substance identification than either unit used separately. It is not possible to make an accurate identification of a particular molecule by gas chromatography or mass spectrometry alone. The mass spectrometry process normally requires a very pure sample while gas chromatography using a traditional detector (e.g. Flame ionization detector) cannot differentiate between multiple molecules that happen to take the same amount of time to travel through the column (''i.e.'' have the same retention time), which results in two or more molecules that co-elute. Sometimes two different molecules can also have a similar pattern of ionized fragments in a mass spectrometer (mass spectrum). Combining the two processes reduces the possibility of error, as it is extremely unlikely that two different molecules will behave in the same way in both a gas chromatograph and a mass spectrometer. Therefore, when an identifying mass spectrum appears at a characteristic retention time in a GC–MS analysis, it typically increases certainty that the analyte of interest is in the sample.
tau.ac.il In this method named cold electron ionization (cold-EI) the molecules exit the GC column, mixed with added helium make up gas and expand into vacuum through a specially designed supersonic nozzle, forming a supersonic molecular beam (SMB). Collisions with the make up gas at the expanding supersonic jet reduce the internal vibrational (and rotational) energy of the analyte molecules, hence reducing the degree of fragmentation caused by the electrons during the ionization process. Cold-EI mass spectra are characterized by an abundant molecular ion while the usual fragmentation pattern is retained, thus making cold-EI mass spectra compatible with library search identification techniques. The enhanced molecular ions increase the identification probabilities of both known and unknown compounds, amplify isomer mass spectral effects and enable the use of isotope abundance analysis for the elucidation of elemental formulas.
Golm Metabolome Database
a mass spectral reference database of plant metabolites {{DEFAULTSORT:Gas Chromatography-Mass Spectrometry Lipid methods Mass spectrometry Chromatography Laboratory techniques Explosive detection
 Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and
Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
to identify different substances within a test sample. Applications of GC–MS include drug
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug i ...
detection, fire
Fire is the rapid oxidation of a fuel in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products.
Flames, the most visible portion of the fire, are produced in the combustion re ...
investigation, environmental analysis, explosives
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An exp ...
investigation, food and flavor analysis, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC–MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace element
__NOTOC__
A trace element is a chemical element of a minute quantity, a trace amount, especially used in referring to a micronutrient, but is also used to refer to minor elements in the composition of a rock, or other chemical substance.
In nutr ...
s in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.
GC–MS has been regarded as a "gold standard
A gold standard is a backed currency, monetary system in which the standard economics, economic unit of account is based on a fixed quantity of gold. The gold standard was the basis for the international monetary system from the 1870s to the ...
" for forensic
Forensic science combines principles of law and science to investigate criminal activity. Through crime scene investigations and laboratory analysis, forensic scientists are able to link suspects to evidence. An example is determining the time and ...
substance identification because it is used to perform a 100% specific
Specific may refer to:
* Specificity (disambiguation)
* Specific, a cure or therapy for a specific illness
Law
* Specific deterrence, focussed on an individual
* Specific finding, intermediate verdict used by a jury in determining the final ...
test, which positively identifies the presence of a particular substance. A nonspecific test merely indicates that any of several in a category of substances is present. Although a nonspecific test could statistically suggest the identity of the substance, this could lead to false positive
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test resu ...
identification. However, the high temperatures (300°C) used in the GC–MS injection port (and oven) can result in thermal degradation of injected molecules, thus resulting in the measurement of degradation products instead of the actual molecule(s) of interest.
History
The first on-line coupling of gas chromatography to a mass spectrometer was reported in the late 1950s. An interest in coupling the methods had been suggested as early as December 1954, but conventional recording techniques had too poor temporal resolution. Fortunately, time-of-flight mass spectrometry developed around the same time allowed to measure spectra thousands times a second. The development of affordable andminiaturized
Miniaturization ( Br.Eng.: ''miniaturisation'') is the trend to manufacture ever-smaller mechanical, optical, and electronic products and devices. Examples include miniaturization of mobile phones, computers and vehicle engine downsizing. In e ...
computer
A computer is a machine that can be Computer programming, programmed to automatically Execution (computing), carry out sequences of arithmetic or logical operations (''computation''). Modern digital electronic computers can perform generic set ...
s has helped in the simplification of the use of this instrument, as well as allowed great improvements in the amount of time it takes to analyze a sample. In 1964, Electronic Associates, Inc. (EAI), a leading U.S. supplier of analog computers, began development of a computer controlled quadrupole mass spectrometer under the direction of Robert E. Finnigan. By 1966 Finnigan and collaborator Mike Uthe's EAI division had sold over 500 quadrupole residual gas-analyzer instruments. In 1967, Finnigan left EAI to form the Finnigan Instrument Corporation along with Roger Sant, T. Z. Chou, Michael Story, Lloyd Friedman, and William Fies. In early 1968, they delivered the first prototype quadrupole GC/MS instruments to Stanford and Purdue University. When Finnigan Instrument Corporation was acquired by Thermo Instrument Systems (later Thermo Fisher Scientific
Thermo Fisher Scientific Inc. is an American life science and clinical research company. It is a global supplier of analytical instruments, clinical development solutions, specialty diagnostics, laboratory, pharmaceutical and biotechnology s ...
) in 1990, it was considered "the world's leading manufacturer of mass spectrometers".
Instrumentation
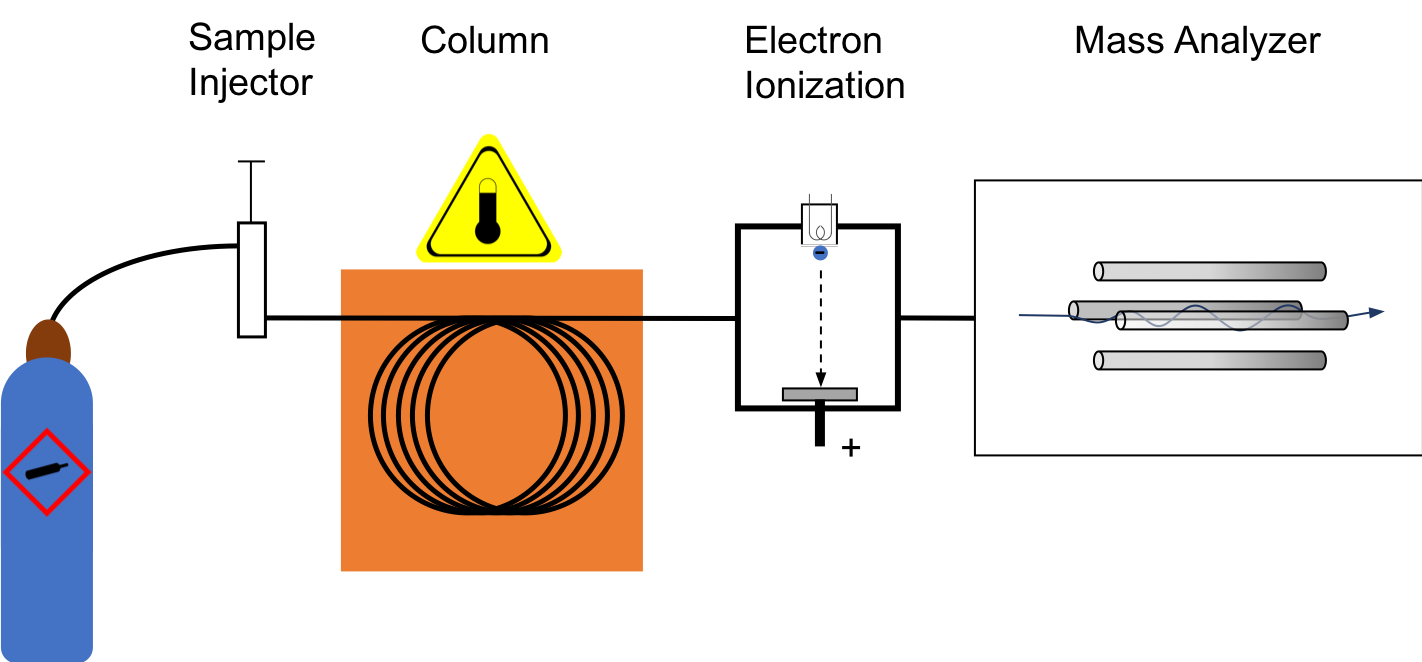
The GC–MS is composed of two major building blocks: the gas chromatograph and themass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is us ...
. The gas chromatograph utilizes a capillary column whose properties regarding molecule separation depend on the column's dimensions (length, diameter, film thickness) as well as the phase properties (e.g. 5% phenyl polysiloxane). The difference in the chemical properties between different molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s in a mixture and their relative affinity for the stationary phase of the column will promote separation of the molecules as the sample travels the length of the column. The molecules are retained by the column and then elute (come off) from the column at different times (called the retention time), and this allows the mass spectrometer downstream to capture, ionize, accelerate, deflect, and detect the ionized molecules separately. The mass spectrometer does this by breaking each molecule into ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ized fragments and detecting these fragments using their mass-to-charge ratio.
 These two components, used together, allow a much finer degree of substance identification than either unit used separately. It is not possible to make an accurate identification of a particular molecule by gas chromatography or mass spectrometry alone. The mass spectrometry process normally requires a very pure sample while gas chromatography using a traditional detector (e.g. Flame ionization detector) cannot differentiate between multiple molecules that happen to take the same amount of time to travel through the column (''i.e.'' have the same retention time), which results in two or more molecules that co-elute. Sometimes two different molecules can also have a similar pattern of ionized fragments in a mass spectrometer (mass spectrum). Combining the two processes reduces the possibility of error, as it is extremely unlikely that two different molecules will behave in the same way in both a gas chromatograph and a mass spectrometer. Therefore, when an identifying mass spectrum appears at a characteristic retention time in a GC–MS analysis, it typically increases certainty that the analyte of interest is in the sample.
These two components, used together, allow a much finer degree of substance identification than either unit used separately. It is not possible to make an accurate identification of a particular molecule by gas chromatography or mass spectrometry alone. The mass spectrometry process normally requires a very pure sample while gas chromatography using a traditional detector (e.g. Flame ionization detector) cannot differentiate between multiple molecules that happen to take the same amount of time to travel through the column (''i.e.'' have the same retention time), which results in two or more molecules that co-elute. Sometimes two different molecules can also have a similar pattern of ionized fragments in a mass spectrometer (mass spectrum). Combining the two processes reduces the possibility of error, as it is extremely unlikely that two different molecules will behave in the same way in both a gas chromatograph and a mass spectrometer. Therefore, when an identifying mass spectrum appears at a characteristic retention time in a GC–MS analysis, it typically increases certainty that the analyte of interest is in the sample.
Purge and trap GC–MS
For the analysis of volatile compounds, a purge and trap (P&T) concentrator system may be used to introduce samples. The target analytes are extracted by mixing the sample with water and purge with inert gas (e.g.Nitrogen gas
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh i ...
) into an airtight chamber, this is known as purging or sparging
Sparging may refer to:
* Sparging (chemistry), a process in which a gas is bubbled through a liquid to remove other gases or volatile compounds
*Air sparging Air sparging, also known as ''in situ'' air stripping and ''in situ'' volatilization is a ...
. The volatile compounds move into the headspace above the water and are drawn along a pressure gradient
In hydrodynamics and hydrostatics, the pressure gradient (typically of air but more generally of any fluid) is a physical quantity that describes in which direction and at what rate the pressure increases the most rapidly around a particular locat ...
(caused by the introduction of the purge gas) out of the chamber. The volatile compounds are drawn along a heated line onto a 'trap'. The trap is a column of adsorbent
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
material at ambient temperature that holds the compounds by returning them to the liquid phase. The trap is then heated and the sample compounds are introduced to the GC–MS column via a volatiles interface, which is a split inlet system. P&T GC–MS is particularly suited to volatile organic compounds
Volatile organic compounds (VOCs) are organic compounds that have a high vapor pressure at room temperature. They are common and exist in a variety of settings and products, not limited to house mold, upholstered furniture, arts and crafts sup ...
(VOCs) and BTEX compounds (aromatic compounds associated with petroleum).
A faster alternative is the "purge-closed loop" system. In this system the inert gas is bubbled through the water until the concentrations of organic compounds in the vapor phase are at equilibrium with concentrations in the aqueous phase. The gas phase is then analysed directly.
Types of mass spectrometer detectors
The most common type of mass spectrometer (MS) associated with a gas chromatograph (GC) is the quadrupole mass spectrometer, sometimes referred to by theHewlett-Packard
The Hewlett-Packard Company, commonly shortened to Hewlett-Packard ( ) or HP, was an American multinational information technology company. It was founded by Bill Hewlett and David Packard in 1939 in a one-car garage in Palo Alto, California ...
(now Agilent) trade name "Mass Selective Detector" (MSD). Another relatively common detector is the ion trap mass spectrometer. Additionally one may find a magnetic sector mass spectrometer, however these particular instruments are expensive and bulky and not typically found in high-throughput service laboratories. Other detectors may be encountered such as time of flight (TOF), tandem quadrupoles (MS-MS) (see below), or in the case of an ion trap MSn where n indicates the number mass spectrometry stages.
GC–tandem MS
When a second phase of mass fragmentation is added, for example using a second quadrupole in a quadrupole instrument, it is called tandem MS (MS/MS). MS/MS can sometimes be used to quantitate low levels of target compounds in the presence of a high sample matrix background. The first quadrupole (Q1) is connected with a collision cell (Q2) and another quadrupole (Q3). Both quadrupoles can be used in scanning or static mode, depending on the type of MS/MS analysis being performed. Types of analysis include product ion scan, precursor ion scan, selected reaction monitoring (SRM) (sometimes referred to as multiple reaction monitoring (MRM)) and neutral loss scan. For example: When Q1 is in static mode (looking at one mass only as in SIM), and Q3 is in scanning mode, one obtains a so-called product ion spectrum (also called "daughter spectrum"). From this spectrum, one can select a prominent product ion which can be the product ion for the chosen precursor ion. The pair is called a "transition" and forms the basis for SRM. SRM is highly specific and virtually eliminates matrix background.Ionization
After the molecules travel the length of the column, pass through the transfer line and enter into the mass spectrometer they are ionized by various methods with typically only one method being used at any given time. Once the sample is fragmented it will then be detected, usually by anelectron multiplier
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary-emissive material, induce emission of roughly 1 to 3 electrons. If an ele ...
, which essentially turns the ionized mass fragment into an electrical signal that is then detected.
The ionization technique chosen is independent of using full scan or SIM.

Electron ionization
By far the most common and perhaps standard form of ionization iselectron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of th ...
(EI). The molecules enter into the MS (the source is a quadrupole or the ion trap itself in an ion trap MS) where they are bombarded with free electrons emitted from a filament, not unlike the filament one would find in a standard light bulb. The electrons bombard the molecules, causing the molecule to fragment in a characteristic and reproducible way. This "hard ionization" technique results in the creation of more fragments of low mass-to-charge ratio (m/z) and few, if any, molecules approaching the molecular mass unit. Hard ionization is considered by mass spectrometrists as the employ of molecular electron bombardment, whereas "soft ionization" is charge by molecular collision with an introduced gas. The molecular fragmentation pattern is dependent upon the electron energy applied to the system, typically 70 eV (electronvolts). The use of 70 eV facilitates comparison of generated spectra with library spectra using manufacturer-supplied software or software developed by the National Institute of Standards (NIST-USA). Spectral library searches employ matching algorithms such as Probability Based Matching and dot-product matching that are used with methods of analysis written by many method standardization agencies. Sources of libraries include NIST, Wiley, the AAFS, and instrument manufacturers.
Cold electron ionization
The "hard ionization" process ofelectron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of th ...
can be softened by the cooling of the molecules before their ionization, resulting in mass spectra that are richer in information.SMB–MS (Supersonic GC–MS)tau.ac.il In this method named cold electron ionization (cold-EI) the molecules exit the GC column, mixed with added helium make up gas and expand into vacuum through a specially designed supersonic nozzle, forming a supersonic molecular beam (SMB). Collisions with the make up gas at the expanding supersonic jet reduce the internal vibrational (and rotational) energy of the analyte molecules, hence reducing the degree of fragmentation caused by the electrons during the ionization process. Cold-EI mass spectra are characterized by an abundant molecular ion while the usual fragmentation pattern is retained, thus making cold-EI mass spectra compatible with library search identification techniques. The enhanced molecular ions increase the identification probabilities of both known and unknown compounds, amplify isomer mass spectral effects and enable the use of isotope abundance analysis for the elucidation of elemental formulas.
Chemical ionization
In chemical ionization (CI) a reagent gas, typicallymethane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
or ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
is introduced into the mass spectrometer. Depending on the technique (positive CI or negative CI) chosen, this reagent gas will interact with the electrons and analyte and cause a 'soft' ionization of the molecule of interest. A softer ionization fragments the molecule to a lower degree than the hard ionization of EI. One of the main benefits of using chemical ionization is that a mass fragment closely corresponding to the molecular weight of the analyte of interest is produced.
In positive chemical ionization (PCI) the reagent gas interacts with the target molecule, most often with a proton exchange. This produces the species in relatively high amounts.
In negative chemical ionization (NCI) the reagent gas decreases the impact of the free electrons on the target analyte. This decreased energy typically leaves the fragment in great supply.
Analysis
A mass spectrometer is typically utilized in one of two ways: full scan or selective ion monitoring (SIM). The typical GC–MS instrument is capable of performing both functions either individually or concomitantly, depending on the setup of the particular instrument. The primary goal of instrument analysis is to quantify an amount of substance. This is done by comparing the relative concentrations among the atomic masses in the generated spectrum. Two kinds of analysis are possible, comparative and original. Comparative analysis essentially compares the given spectrum to a spectrum library to see if its characteristics are present for some sample in the library. This is best performed by acomputer
A computer is a machine that can be Computer programming, programmed to automatically Execution (computing), carry out sequences of arithmetic or logical operations (''computation''). Modern digital electronic computers can perform generic set ...
because there are a myriad of visual distortions that can take place due to variations in scale. Computers can also simultaneously correlate more data (such as the retention times identified by GC), to more accurately relate certain data. Deep learning was shown to lead to promising results in the identification of VOCs from raw GC–MS data.
Another method of analysis measures the peaks in relation to one another. In this method, the tallest peak is assigned 100% of the value, and the other peaks being assigned proportionate values. All values above 3% are assigned. The total mass of the unknown compound is normally indicated by the parent peak. The value of this parent peak can be used to fit with a chemical formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
containing the various elements which are believed to be in the compound. The isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
pattern in the spectrum, which is unique for elements that have many natural isotopes, can also be used to identify the various elements present. Once a chemical formula has been matched to the spectrum, the molecular structure and bonding can be identified, and must be consistent with the characteristics recorded by GC–MS. Typically, this identification is done automatically by programs which come with the instrument, given a list of the elements which could be present in the sample.
A "full spectrum" analysis considers all the "peaks" within a spectrum. Conversely, selective ion monitoring (SIM) only monitors selected ions associated with a specific substance. This is done on the assumption that at a given retention time, a set of ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s is characteristic of a certain compound. This is a fast and efficient analysis, especially if the analyst has previous information about a sample or is only looking for a few specific substances. When the amount of information collected about the ions in a given gas chromatographic peak decreases, the sensitivity of the analysis increases. So, SIM analysis allows for a smaller quantity of a compound to be detected and measured, but the degree of certainty about the identity of that compound is reduced.
Full scan MS
When collecting data in the full scan mode, a target range of mass fragments is determined and put into the instrument's method. An example of a typical broad range of mass fragments to monitor would be ''m/z'' 50 to ''m/z'' 400. The determination of what range to use is largely dictated by what one anticipates being in the sample while being cognizant of the solvent and other possible interferences. A MS should not be set to look for mass fragments too low or else one may detect air (found as ''m/z'' 28 due to nitrogen), carbon dioxide (''m/z'' 44) or other possible interference. Additionally if one is to use a large scan range then sensitivity of the instrument is decreased due to performing fewer scans per second since each scan will have to detect a wide range of mass fragments. Full scan is useful in determining unknown compounds in a sample. It provides more information than SIM when it comes to confirming or resolving compounds in a sample. During instrument method development it may be common to first analyze test solutions in full scan mode to determine the retention time and the mass fragment fingerprint before moving to a SIM instrument method.Selective ion monitoring
In selective ion monitoring (SIM) certain ion fragments are entered into the instrument method and only those mass fragments are detected by the mass spectrometer. The advantages of SIM are that the detection limit is lower since the instrument is only looking at a small number of fragments (e.g. three fragments) during each scan. More scans can take place each second. Since only a few mass fragments of interest are being monitored, matrix interferences are typically lower. To additionally confirm the likelihood of a potentially positive result, it is relatively important to be sure that the ion ratios of the various mass fragments are comparable to a known reference standard.Applications
Environmental monitoring and cleanup
GC–MS is becoming the tool of choice for tracking organic pollutants in the environment. The cost of GC–MS equipment has decreased significantly, and the reliability has increased at the same time, which has contributed to its increased adoption inenvironmental studies
Environmental studies (EVS or EVST) is a multidisciplinary academic field which systematically studies human behavior, human interaction with the Natural environment, environment. Environmental studies connects principles from the physical sci ...
.
Criminal forensics
GC–MS can analyze the particles from a human body in order to help link a criminal to acrime
In ordinary language, a crime is an unlawful act punishable by a State (polity), state or other authority. The term ''crime'' does not, in modern criminal law, have any simple and universally accepted definition,Farmer, Lindsay: "Crime, definiti ...
. The analysis of fire
Fire is the rapid oxidation of a fuel in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products.
Flames, the most visible portion of the fire, are produced in the combustion re ...
debris using GC–MS is well established, and there is even an established American Society for Testing and Materials (ASTM) standard for fire debris analysis. GCMS/MS is especially useful here as samples often contain very complex matrices, and results used in court need to be highly accurate.
Law enforcement
GC–MS is increasingly used for detection of illegal narcotics, and may eventually supplant drug-sniffing dogs. /sup> A simple and selective GC–MS method for detecting marijuana usage was recently developed by the Robert Koch Institute in Germany. It involves identifying an acid metabolite of tetrahydrocannabinol (THC), the active ingredient in marijuana, in urine samples by employing derivatization in the sample preparation. GC–MS is also commonly used in forensic toxicology to find drugs and/or poisons in biological specimens of suspects, victims, or the deceased. In drug screening, GC–MS methods frequently utilize liquid-liquid extraction as a part of sample preparation, in which target compounds are extracted from blood plasma.Sports anti-doping analysis
GC–MS is the main tool used in sports anti-doping laboratories to test athletes' urine samples for prohibited performance-enhancing drugs, for exampleanabolic steroids
Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are a class of drugs that are structurally related to testosterone, the main male sex hormone, and produce effects by binding to the androgen receptor (AR). Anabolic steroids ...
.
Security
A post–September 11 development,explosive detection
Explosive detection is a non-destructive inspection process to determine whether a container contains explosive material. Explosive detection is commonly used at airports, ports and for border control.
Detection tools
Colorimetrics & automated ...
systems have become a part of all US airport
An airport is an aerodrome with extended facilities, mostly for commercial Aviation, air transport. They usually consist of a landing area, which comprises an aerially accessible open space including at least one operationally active surf ...
s. These systems run on a host of technologies, many of them based on GC–MS. There are only three manufacturers certified by the FAA to provide these systems, one of which is Thermo Detection (formerly Thermedics), which produces the EGIS, a GC–MS-based line of explosives detectors. The other two manufacturers are Barringer Technologies, now owned by Smith's Detection Systems, and Ion Track Instruments, part of General Electric Infrastructure Security Systems.
Chemical warfare agent detection
As part of the post-September 11 drive towards increased capability in homeland security and public health preparedness, traditional GC–MS units with transmission quadrupole mass spectrometers, as well as those with cylindrical ion trap (CIT-MS) and toroidal ion trap (T-ITMS) mass spectrometers have been modified for field portability and near real-time detection of chemical warfare agents (CWA) such as sarin, soman, and VX. These complex and large GC–MS systems have been modified and configured with resistively heated low thermal mass (LTM) gas chromatographs that reduce analysis time to less than ten percent of the time required in traditional laboratory systems. Additionally, the systems are smaller, and more mobile, including units that are mounted in mobile analytical laboratories (MAL), such as those used by the United States Marine Corps Chemical and Biological Incident Response Force MAL and other similar laboratories, and systems that are hand-carried by two-person teams or individuals, much ado to the smaller mass detectors. Depending on the system, the analytes can be introduced via liquid injection, desorbed from sorbent tubes through a thermal desorption process, or with solid-phase micro extraction (SPME).Chemical engineering
GC–MS is used for the analysis of unknown organic compound mixtures. One critical use of this technology is the use of GC–MS to determine the composition of bio-oils processed from raw biomass. GC–MS is also utilized in the identification of continuous phase component in a smart material, magnetorheological (MR) fluid.Food, beverage and perfume analysis
Food
Food is any substance consumed by an organism for Nutrient, nutritional support. Food is usually of plant, animal, or Fungus, fungal origin and contains essential nutrients such as carbohydrates, fats, protein (nutrient), proteins, vitamins, ...
s and beverage
A drink or beverage is a liquid intended for human consumption. In addition to their basic function of satisfying thirst, drinks play important roles in human culture. Common types of drinks include plain drinking water, milk, juice, smoothie ...
s contain numerous aromatic compounds, some naturally present in the raw materials and some forming during processing. GC–MS is extensively used for the analysis of these compounds which include esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
, fatty acids
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
, alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
, aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
, terpenes
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...
etc. It is also used to detect and measure contaminants from spoilage or adulteration
An adulterant is a substance secretly added to another that may compromise the safety or effectiveness. Typical substances that are adulterated include food, cosmetics, pharmaceuticals or fuels.
Definition
Adulteration is the practice of secre ...
which may be harmful and which is often controlled by governmental agencies, for example pesticides
Pesticides are substances that are used to pest control, control pest (organism), pests. They include herbicides, insecticides, nematicides, fungicides, and many others (see table). The most common of these are herbicides, which account for a ...
.
Astrochemistry
Several GC–MS systems have left earth. Two were brought toMars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
by the Viking program
The ''Viking'' program consisted of a pair of identical American space probes, ''Viking 1'' and ''Viking 2'' both launched in 1975, and landed on Mars in 1976. The mission effort began in 1968 and was managed by the NASA Langley Research Cent ...
. Venera
The Venera (, 'Venus') program was a series of space probes developed by the Soviet Union between 1961 and 1984 to gather information about the planet Venus.
Thirteen probes successfully entered the Venusian atmosphere, including the two ...
11 and 12 and Pioneer Venus analysed the atmosphere of Venus
Venus is the second planet from the Sun. It is often called Earth's "twin" or "sister" planet for having almost the same size and mass, and the closest orbit to Earth's. While both are rocky planets, Venus has an atmosphere much thicker ...
with GC–MS. The Huygens probe of the Cassini–Huygens
''Cassini–Huygens'' ( ), commonly called ''Cassini'', was a space research, space-research mission by NASA, the European Space Agency (ESA), and the Italian Space Agency (ASI) to send a space probe to study the planet Saturn and its system, i ...
mission landed one GC–MS on Saturn
Saturn is the sixth planet from the Sun and the second largest in the Solar System, after Jupiter. It is a gas giant, with an average radius of about 9 times that of Earth. It has an eighth the average density of Earth, but is over 95 tim ...
's largest moon, Titan
Titan most often refers to:
* Titan (moon), the largest moon of Saturn
* Titans, a race of deities in Greek mythology
Titan or Titans may also refer to:
Arts and entertainment
Fictional entities
Fictional locations
* Titan in fiction, fictiona ...
. The MSL Curiosity rover's Sample analysis at Mars (SAM) instrument contains both a gas chromatograph and quadrupole mass spectrometer that can be used in tandem as a GC–MS. The material in the comet
A comet is an icy, small Solar System body that warms and begins to release gases when passing close to the Sun, a process called outgassing. This produces an extended, gravitationally unbound atmosphere or Coma (cometary), coma surrounding ...
67P/Churyumov–Gerasimenko
67P/Churyumov–Gerasimenko (abbreviated as 67P or 67P/C–G) is a Jupiter-family comet. It is originally from the Kuiper belt and has an orbital period of 6.45 years as of 2012, a rotation period of approximately 12.4 hours, and a maximum velo ...
was analysed by the Rosetta
Rosetta ( ) or Rashid (, ; ) is a port city of the Nile Delta, east of Alexandria, in Egypt's Beheira governorate. The Rosetta Stone was discovered there in 1799.
Founded around the 9th century on the site of the ancient town of Bolbitine, R ...
mission with a chiral GC–MS in 2014.
Medicine
Dozens of congenital metabolic diseases also known as inborn errors of metabolism (IEM) are now detectable bynewborn screening
Newborn screening (NBS) is a public health program of screening (medicine), screening in infants shortly after birth for conditions that are treatable, but not clinically evident in the newborn period. The goal is to identify infants at risk for ...
tests, especially the testing using gas chromatography–mass spectrometry. GC–MS can determine compounds in urine even in minor concentration. These compounds are normally not present but appear in individuals suffering with metabolic disorders. This is increasingly becoming a common way to diagnose IEM for earlier diagnosis and institution of treatment eventually leading to a better outcome. It is now possible to test a newborn for over 100 genetic metabolic disorders by a urine test at birth based on GC–MS.
In combination with isotopic labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through chemical reaction, metabolic pathway, or a biological cell. The reactant is 'labeled' ...
of metabolic compounds, the GC–MS is used for determining metabolic activity
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
. Most applications are based on the use of 13C as the labeling and the measurement of 13C-12C ratios with an isotope ratio mass spectrometer (IRMS); an MS with a detector designed to measure a few select ions and return values as ratios.
See also
*Capillary electrophoresis–mass spectrometry
Capillary electrophoresis–mass spectrometry (CE–MS) is an analytical chemistry technique formed by the combination of the liquid separation process of capillary electrophoresis with mass spectrometry. CE–MS combines advantages of both CE an ...
* Ion-mobility spectrometry–mass spectrometry
* Liquid chromatography–mass spectrometry
* Prolate trochoidal mass spectrometer
* Pyrolysis–gas chromatography–mass spectrometry
References
Bibliography
* * * * * * * * * *External links
*Golm Metabolome Database
a mass spectral reference database of plant metabolites {{DEFAULTSORT:Gas Chromatography-Mass Spectrometry Lipid methods Mass spectrometry Chromatography Laboratory techniques Explosive detection