Electron diffraction is a generic term for phenomena associated with changes in the direction of
electron beams due to
elastic
Elastic is a word often used to describe or identify certain types of elastomer, Elastic (notion), elastic used in garments or stretch fabric, stretchable fabrics.
Elastic may also refer to:
Alternative name
* Rubber band, ring-shaped band of rub ...
interactions with
atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
. It occurs due to
elastic scattering
Elastic scattering is a form of particle scattering in scattering theory, nuclear physics and particle physics. In this process, the internal states of the Elementary particle, particles involved stay the same. In the non-relativistic case, where ...
, when there is no change in the energy of the electrons.
The negatively charged electrons are scattered due to
Coulomb forces when they interact with both the positively charged atomic core and the negatively charged electrons around the atoms. The resulting map of the directions of the electrons far from the sample is called a diffraction pattern, see for instance
Figure 1. Beyond patterns showing the directions of electrons, electron diffraction also plays a major role in the contrast of images in
electron microscope
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing it ...
s.
This article provides an overview of electron diffraction and electron diffraction patterns, collective referred to by the generic name electron diffraction. This includes aspects of how in a
general way electrons can act as waves, and diffract and interact with matter. It also involves the extensive
history
History is the systematic study of the past, focusing primarily on the Human history, human past. As an academic discipline, it analyses and interprets evidence to construct narratives about what happened and explain why it happened. Some t ...
behind modern electron diffraction, how the combination of developments in the 19th century in understanding and controlling
electrons in vacuum and the early 20th century developments with
electron waves were combined with early
instruments, giving birth to electron microscopy and diffraction in 1920–1935. While this was the birth, there have been a large number of
further developments since then.
There are many
types and techniques of electron diffraction. The most common approach is where the electrons
transmit through a thin sample, from 1 nm to 100 nm (10 to 1000 atoms thick), where the results depending upon how the atoms are arranged in the material, for instance a
single crystal
In materials science, a single crystal (or single-crystal solid or monocrystalline solid) is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no Grain boundary, grain bound ...
,
many crystals or
different types of solids. Other cases such as
larger repeats,
no periodicity or
disorder have their own characteristic patterns. There are many different ways of collecting diffraction information, from parallel illumination to a
converging beam of electrons or where the beam is
rotated or
scanned across the sample which produce information that is often easier to interpret. There are also many other types of instruments. For instance, in
a scanning electron microscope (SEM),
electron backscatter diffraction can be used to determine crystal orientation across the sample. Electron diffraction patterns can also be used to characterize molecules using
gas electron diffraction, liquids, surfaces using lower energy electrons, a technique called
LEED, and by reflecting electrons off surfaces, a technique called
RHEED.
There are also many levels of analysis of electron diffraction, including:
# The simplest approximation using the de Broglie wavelength
for electrons, where only the
geometry
Geometry (; ) is a branch of mathematics concerned with properties of space such as the distance, shape, size, and relative position of figures. Geometry is, along with arithmetic, one of the oldest branches of mathematics. A mathematician w ...
is considered and often
Bragg's law is invoked. This approach only considers the electrons far from the sample, a far-field or
Fraunhofer approach.
# The first level of more accuracy where it is approximated that the electrons are only scattered once, which is called
kinematical diffraction and is also a far-field or Fraunhofer
approach.
# More complete and accurate explanations where multiple scattering is included, what is called
dynamical diffraction (e.g. refs
). These involve more general analyses using relativistically corrected
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
methods, and track the electrons through the sample, being accurate both near and far from the sample (both
Fresnel and
Fraunhofer diffraction).
Electron diffraction is similar to
x-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
and
neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of Neutron temperature, thermal or ...
. However, unlike x-ray and neutron diffraction where the simplest approximations are quite accurate, with electron diffraction this is not the case.
Simple models give the geometry of the intensities in a diffraction pattern, but dynamical diffraction approaches are needed for accurate intensities and the positions of diffraction spots.
A primer on electron diffraction
All matter can be thought of as
matter wave
Matter waves are a central part of the theory of quantum mechanics, being half of wave–particle duality. At all scales where measurements have been practical, matter exhibits wave-like behavior. For example, a beam of electrons can be diffract ...
s,
from small particles such as electrons up to macroscopic objects – although it is impossible to measure any of the "wave-like" behavior of macroscopic objects. Waves can move around objects and create interference patterns,
[
] and a classic example is the
Young's two-slit experiment shown in
Figure 2, where a wave impinges upon two slits in the first of the two images (blue waves). After going through the slits there are directions where the wave is stronger, ones where it is weaker – the wave has been
diffracted.
If instead of two slits there are a number of small points then similar phenomena can occur as shown in the second image where the wave (red and blue) is coming in from the bottom right corner. This is comparable to diffraction of an
electron wave where the small dots would be atoms in a small crystal, see also note. Note the strong dependence on the relative orientation of the crystal and the incoming wave.
Close to an aperture or atoms, often called the "sample", the electron wave would be described in terms of near field or
Fresnel diffraction
In optics, the Fresnel diffraction equation for near-field diffraction is an approximation of the Kirchhoff's diffraction formula, Kirchhoff–Fresnel diffraction that can be applied to the propagation of waves in the near and far field, near fi ...
.
This has relevance for imaging within
electron microscope
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing it ...
s,
whereas electron diffraction patterns are measured far from the sample, which is described as far-field or Fraunhofer diffraction.
A map of the directions of the
electron waves leaving the sample will show high intensity (white) for favored directions, such as the three prominent ones in the Young's two-slit experiment of
Figure 2, while the other directions will be low intensity (dark). Often there will be an array of spots (preferred directions) as in
Figure 1 and the other figures shown later.
History
The historical background is divided into several subsections. The first is the general background to electrons in vacuum and the technological developments that led to
cathode-ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
s as well as
vacuum tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It ...
s that dominated early television and electronics; the second is how these led to the development of electron microscopes; the last is work on the nature of electron beams and the fundamentals of how electrons behave, a key component of
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
and the explanation of electron diffraction.
Electrons in vacuum
Experiments involving electron beams occurred long before the discovery of the electron;
ēlektron (ἤλεκτρον) is the Greek word for
amber
Amber is fossilized tree resin. Examples of it have been appreciated for its color and natural beauty since the Neolithic times, and worked as a gemstone since antiquity."Amber" (2004). In Maxine N. Lurie and Marc Mappen (eds.) ''Encyclopedia ...
,
[
] which is connected to the recording of electrostatic charging
by
Thales of Miletus
Thales of Miletus ( ; ; ) was an Ancient Greek pre-Socratic philosopher from Miletus in Ionia, Asia Minor. Thales was one of the Seven Sages, founding figures of Ancient Greece.
Beginning in eighteenth-century historiography, many came to ...
around 585 BCE, and possibly others even earlier.
In 1650,
Otto von Guericke
Otto von Guericke ( , , ; spelled Gericke until 1666; – ) was a German scientist, inventor, mathematician and physicist. His pioneering scientific work, the development of experimental methods and repeatable demonstrations on the physics of ...
invented the
vacuum pump
A vacuum pump is a type of pump device that draws gas particles from a sealed volume in order to leave behind a partial vacuum. The first vacuum pump was invented in 1650 by Otto von Guericke, and was preceded by the suction pump, which dates to ...
[
] allowing for the study of the effects of high voltage electricity passing through
rarefied air. In 1838,
Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
applied a high voltage between two metal
electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s at either end of a glass tube that had been partially evacuated of air, and noticed a strange light arc with its beginning at the
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
(negative electrode) and its end at the
anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
(positive electrode).
[Michael Faraday (1838]
"VIII. Experimental researches in electricity. — Thirteenth series.,"
''Philosophical Transactions of the Royal Society of London'', 128 : 125–168. Building on this, in the 1850s,
Heinrich Geissler was able to achieve a pressure of around 10
−3 atmospheres, inventing what became known as
Geissler tubes. Using these tubes, while studying electrical conductivity in
rarefied gases in 1859,
Julius Plücker
Julius Plücker (16 June 1801 – 22 May 1868) was a German mathematician and physicist. He made fundamental contributions to the field of analytical geometry and was a pioneer in the investigations of cathode rays that led eventually to the di ...
observed that the radiation emitted from the negatively charged cathode caused phosphorescent light to appear on the tube wall near it, and the region of the phosphorescent light could be moved by application of a magnetic field.
In 1869, Plücker's student
Johann Wilhelm Hittorf found that a solid body placed between the cathode and the phosphorescence would cast a shadow on the tube wall, e.g.
Figure 3.
[
] Hittorf inferred that there are straight rays emitted from the cathode and that the phosphorescence was caused by the rays striking the tube walls. In 1876
Eugen Goldstein showed that the rays were emitted perpendicular to the cathode surface, which differentiated them from the incandescent light.
Eugen Goldstein dubbed them
cathode ray
Cathode rays are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the c ...
s.
[
] By the 1870s
William Crookes
Sir William Crookes (; 17 June 1832 – 4 April 1919) was an English chemist and physicist who attended the Royal College of Chemistry, now part of Imperial College London, and worked on spectroscopy. He was a pioneer of vacuum tubes, inventing ...
and others were able to evacuate glass tubes below 10
−6 atmospheres, and observed that the glow in the tube disappeared when the pressure was reduced but the glass behind the anode began to glow. Crookes was also able to show that the particles in the cathode rays were negatively charged and could be deflected by an electromagnetic field.
In 1897,
Joseph Thomson measured the mass of these cathode rays, proving they were made of particles. These particles, however, were 1800 times lighter than the lightest particle known at that time – a
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atom. These were originally called ''corpuscles'' and later named electrons by
George Johnstone Stoney
George Johnstone Stoney (15 February 1826 – 5 July 1911) was an Irish physicist known for introducing the term ''electron'' as the "fundamental unit quantity of electricity". He initially named it ''electrolion'' in 1881, and later named it ...
.
The control of electron beams that this work led to resulted in significant technology advances in electronic amplifiers and television displays.
Waves, diffraction and quantum mechanics
Independent of the developments for electrons in vacuum, at about the same time the components of quantum mechanics were being assembled. In 1924
Louis de Broglie in his PhD thesis ''Recherches sur la théorie des quanta''
introduced his theory of
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
waves. He suggested that an electron around a nucleus could be thought of as
standing wave
In physics, a standing wave, also known as a stationary wave, is a wave that oscillates in time but whose peak amplitude profile does not move in space. The peak amplitude of the wave oscillations at any point in space is constant with respect t ...
s,
and that electrons and all matter could be considered as waves. He merged the idea of thinking about them as particles (or corpuscles), and of thinking of them as waves. He proposed that particles are bundles of waves (
wave packet
In physics, a wave packet (also known as a wave train or wave group) is a short burst of localized wave action that travels as a unit, outlined by an Envelope (waves), envelope. A wave packet can be analyzed into, or can be synthesized from, a ...
s) that move with a
group velocity
The group velocity of a wave is the velocity with which the overall envelope shape of the wave's amplitudes—known as the ''modulation'' or ''envelope (waves), envelope'' of the wave—propagates through space.
For example, if a stone is thro ...
and have an
effective mass, see for instance
Figure 4. Both of these depend upon the energy, which in turn connects to the
wavevector
In physics, a wave vector (or wavevector) is a vector used in describing a wave, with a typical unit being cycle per metre. It has a magnitude and direction. Its magnitude is the wavenumber of the wave (inversely proportional to the wavelength), ...
and the relativistic formulation of
Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
a few years before.
This rapidly became part of what was called by
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
''undulatory mechanics'',
now called the
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
or wave mechanics. As stated by
Louis de Broglie on September 8, 1927, in the preface to the German translation of his theses (in turn translated into English):
''M. Einstein from the beginning has supported my thesis, but it was M. E. Schrödinger who developed the propagation equations of a new theory and who in searching for its solutions has established what has become known as “Wave Mechanics”.''
The Schrödinger equation combines the kinetic energy of waves and the potential energy due to, for electrons, the
Coulomb potential. He was able to explain earlier work such as the quantization of the energy of electrons around atoms in the
Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model was a model of the atom that incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear Rutherford model, model, i ...
, as well as many other phenomena.
Electron waves as hypothesized
by de Broglie were automatically part of the solutions to his equation,
see also
introduction to quantum mechanics and
matter waves.
Both the wave nature and the undulatory mechanics approach were experimentally confirmed for electron beams by experiments from two groups performed independently, the first the
Davisson–Germer experiment,
the other by
George Paget Thomson and Alexander Reid; see note for more discussion. Alexander Reid, who was Thomson's graduate student, performed the first experiments, but he died soon after in a motorcycle accident and is rarely mentioned. These experiments were rapidly followed by the first non-relativistic diffraction model for electrons by
Hans Bethe based upon the Schrödinger equation,
which is very close to how electron diffraction is now described. Significantly,
Clinton Davisson and
Lester Germer noticed
that their results could not be interpreted using a
Bragg's law approach as the positions were systematically different; the approach of
Hans Bethe which includes the refraction due to the average potential yielded more accurate results. These advances in understanding of electron wave mechanics were important for many developments of electron-based analytical techniques such as
Seishi Kikuchi's observations of lines due to combined elastic and inelastic scattering,
developed by
Herman Mark and Raymond Weil, diffraction in liquids by Louis Maxwell,
and the first electron microscopes developed by
Max Knoll and
Ernst Ruska.
Electron microscopes and early electron diffraction
In order to have a practical microscope or diffractometer, just having an electron beam was not enough, it needed to be controlled. Many developments laid the groundwork of
electron optics; see the paper by Chester J. Calbick for an overview of the early work. One significant step was the work of
Heinrich Hertz
Heinrich Rudolf Hertz (; ; 22 February 1857 – 1 January 1894) was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwell's equations of electromagnetism.
Biography
Heinri ...
in 1883 who made a cathode-ray tube with electrostatic and magnetic deflection, demonstrating manipulation of the direction of an electron beam. Others were focusing of electrons by an axial magnetic field by
Emil Wiechert in 1899, improved oxide-coated cathodes which produced more electrons by
Arthur Wehnelt in 1905 and the development of the electromagnetic lens in 1926 by
Hans Busch.

Building an electron microscope involves combining these elements, similar to an
optical microscope
The optical microscope, also referred to as a light microscope, is a type of microscope that commonly uses visible light and a system of lenses to generate magnified images of small objects. Optical microscopes are the oldest design of micros ...
but with magnetic or electrostatic lenses instead of glass ones. To this day the issue of who invented the transmission electron microscope is controversial, as discussed by Thomas Mulvey
[ and more recently by Yaping Tao. Extensive additional information can be found in the articles by Martin Freundlich, Reinhold Rüdenberg][.] and Mulvey.Technische Hochschule
A ''Technische Hochschule'' (, plural: ''Technische Hochschulen'', abbreviated ''TH'') is a type of university focusing on engineering sciences in Germany. Previously, it also existed in Austria, Switzerland, the Netherlands (), and Finland (, ) ...
in Charlottenburg (now Technische Universität Berlin
(TU Berlin; also known as Berlin Institute of Technology and Technical University of Berlin, although officially the name should not be translated) is a public university, public research university located in Berlin, Germany. It was the first ...
), (Professor of High Voltage Technology and Electrical Installations) appointed Max Knoll to lead a team of researchers to advance research on electron beams and cathode-ray oscilloscopes. The team consisted of several PhD students including Ernst Ruska. In 1931, Max Knoll and Ernst RuskaNobel Prize in Physics
The Nobel Prize in Physics () is an annual award given by the Royal Swedish Academy of Sciences for those who have made the most outstanding contributions to mankind in the field of physics. It is one of the five Nobel Prizes established by the ...
in 1986.)
Apparently independent of this effort was work at Siemens-Schuckert by Reinhold Rudenberg. According to patent law (U.S. Patent No. 2058914 and 2070318, both filed in 1932), he is the inventor of the electron microscope, but it is not clear when he had a working instrument. He stated in a very brief article in 1932 that Siemens had been working on this for some years before the patents were filed in 1932, so his effort was parallel to the university effort. He died in 1961, so similar to Max Knoll, was not eligible for a share of the Nobel Prize.
These instruments could produce magnified images, but were not particularly useful for electron diffraction; indeed, the wave nature of electrons was not exploited during the development. Key for electron diffraction in microscopes was the advance in 1936 where showed that they could be used as micro-diffraction cameras with an aperture—the birth of selected area electron diffraction.[ As early as 1929 Germer investigated gas adsorption, and in 1932 Harrison E. Farnsworth probed single crystals of copper and silver. However, the vacuum systems available at that time were not good enough to properly control the surfaces, and it took almost forty years before these became available.]
Subsequent developments in methods and modelling
Despite early successes such as the determination of the positions of hydrogen atoms in NH4Cl crystals by W. E. Laschkarew and I. D. Usykin in 1933, boric acid by John M. Cowley in 1953''Thus was founded the belief, amounting in some cases almost to an article of faith, and persisting even to the present day, that it is impossible to interpret the intensities of electron diffraction patterns to gain structural information.''
This has changed, in transmission, reflection and for low energies. Some of the key developments (some of which are also described later) from the early days to 2023 have been:
* Fast numerical methods based upon the Cowley–Moodie multislice algorithm,[ it was extended by Peter Goodman and Gunter Lehmpfuhl,]point group
In geometry, a point group is a group (mathematics), mathematical group of symmetry operations (isometry, isometries in a Euclidean space) that have a Fixed point (mathematics), fixed point in common. The Origin (mathematics), coordinate origin o ...
s and space group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that ...
s. It can also be used for higher-level refinements of the electron density; for a brief history see CBED history. In many cases this is the best method to determine symmetry.surface science
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid– gas interfaces, solid– vacuum interfaces, and liquid– gas interfaces. It includes the ...
.charge-coupled device
A charge-coupled device (CCD) is an integrated circuit containing an array of linked, or coupled, capacitors. Under the control of an external circuit, each capacitor can transfer its electric charge to a neighboring capacitor. CCD sensors are a ...
Core elements of electron diffraction
Plane waves, wavevectors and reciprocal lattice
What is seen in an electron diffraction pattern depends upon the sample and also the energy of the electrons. The electrons need to be considered as waves, which involves describing the electron via a wavefunction, written in crystallographic notation (see notes and) as:quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
description; one cannot use a classical approach. The vector is called the wavevector, has units of inverse nanometers, and the form above is called a plane wave as the term inside the exponential is constant on the surface of a plane. The vector is what is used when drawing ray diagrams,Dirac equation
In particle physics, the Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin-1/2 massive particles, called "Dirac ...
, which as spin does not normally matter can be reduced to the Klein–Gordon equation. Fortunately one can side-step many complications and use a non-relativistic approach based around the Schrödinger equation.Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
, is a relativistic effective mass used to cancel out the relativistic terms for electrons of energy with the speed of light and the rest mass of the electron. The concept of effective mass occurs throughout physics (see for instance Ashcroft and Mermin),electron hole
In physics, chemistry, and electronic engineering, an electron hole (often simply called a hole) is a quasiparticle denoting the lack of an electron at a position where one could exist in an atom or crystal structure, atomic lattice. Since in ...
, which acts as if it is a particle with a positive charge and a mass similar to that of an electron, although it can be several times lighter or heavier. For electron diffraction the electrons behave as if they are non-relativistic particles of mass in terms of how they interact with the atoms.electronvolt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an Voltage, electric potential difference of one volt in vacuum ...
s (eV), the voltage used to accelerate the electrons; the actual energy of each electron is this voltage times the electron charge. For context, the typical energy of a chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
is a few eV; electron diffraction involves electrons up to .
The magnitude of the interaction of the electrons with a material scales asFourier series
A Fourier series () is an Series expansion, expansion of a periodic function into a sum of trigonometric functions. The Fourier series is an example of a trigonometric series. By expressing a function as a sum of sines and cosines, many problems ...
(see for instance Ashcroft and Mermin),reciprocal lattice
Reciprocal lattice is a concept associated with solids with translational symmetry which plays a major role in many areas such as X-ray and electron diffraction as well as the energies of electrons in a solid. It emerges from the Fourier tran ...
vector and the corresponding Fourier coefficient of the potential. The reciprocal lattice vector is often referred to in terms of Miller indices , a sum of the individual reciprocal lattice vectors with integers in the form:Fourier transform
In mathematics, the Fourier transform (FT) is an integral transform that takes a function as input then outputs another function that describes the extent to which various frequencies are present in the original function. The output of the tr ...
of the shape of the object. If, for instance, the object is small in one dimension then the shape function extends far in that direction in the Fourier transform—a reciprocal relationship.
 Around each reciprocal lattice point one has this shape function.
Around each reciprocal lattice point one has this shape function.
Kinematical diffraction
In Kinematical theory an approximation is made that the electrons are only scattered once.reciprocal lattice
Reciprocal lattice is a concept associated with solids with translational symmetry which plays a major role in many areas such as X-ray and electron diffraction as well as the energies of electrons in a solid. It emerges from the Fourier tran ...
vector, is a simplified form of the Debye–Waller factor,x-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
or neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of Neutron temperature, thermal or ...
because they interact with matter far less and often Bragg's law
Dynamical diffraction
While kinematical diffraction is adequate to understand the geometry of the diffraction spots, it does not correctly give the intensities and has a number of other limitations. For a more complete approach one has to include multiple scattering of the electrons using methods that date back to the early work of Hans Bethe in 1928. The main components of current dynamical diffraction of electrons include:
* Taking into account the scattering back into the incident beam both from diffracted beams and between all others, not just single scattering from the incident beam to diffracted beams.
The main components of current dynamical diffraction of electrons include:
* Taking into account the scattering back into the incident beam both from diffracted beams and between all others, not just single scattering from the incident beam to diffracted beams.[ matrix methods]crystallographic defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in Crystal, crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the Crysta ...
s, and also what Jens Lindhard called the string potential.
* For transmission electron microscopes effects due to variations in the thickness of the sample and the normal to the surface.[
]
Kikuchi lines
Kikuchi lines,elastic scattering
Elastic scattering is a form of particle scattering in scattering theory, nuclear physics and particle physics. In this process, the internal states of the Elementary particle, particles involved stay the same. In the non-relativistic case, where ...
, and also scattered inelastically losing part of their energy. These occur simultaneously, and cannot be separated – according to the Copenhagen interpretation of quantum mechanics, only the probabilities of electrons at detectors can be measured. Kikuchi lines come in pairs forming Kikuchi bands, and are indexed in terms of the crystallographic planes they are connected to, with the angular width of the band equal to the magnitude of the corresponding diffraction vector . The position of Kikuchi bands is fixed with respect to each other and the orientation of the sample, but not against the diffraction spots or the direction of the incident electron beam. As the crystal is tilted, the bands move on the diffraction pattern.
Kikuchi lines come in pairs forming Kikuchi bands, and are indexed in terms of the crystallographic planes they are connected to, with the angular width of the band equal to the magnitude of the corresponding diffraction vector . The position of Kikuchi bands is fixed with respect to each other and the orientation of the sample, but not against the diffraction spots or the direction of the incident electron beam. As the crystal is tilted, the bands move on the diffraction pattern.
Types and techniques
In a transmission electron microscope
 Electron diffraction in a TEM exploits controlled electron beams using electron optics.
Electron diffraction in a TEM exploits controlled electron beams using electron optics.crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
.
It is common to combine it with other methods, for instance images using selected diffraction beams, high-resolution images showing the atomic structure, chemical analysis through energy-dispersive x-ray spectroscopy, investigations of electronic structure and bonding through electron energy loss spectroscopy, and studies of the electrostatic potential through electron holography; this list is not exhaustive. Compared to x-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
, TEM analysis is significantly more localized and can be used to obtain information from tens of thousands of atoms to just a few or even single atoms.
Formation of a diffraction pattern
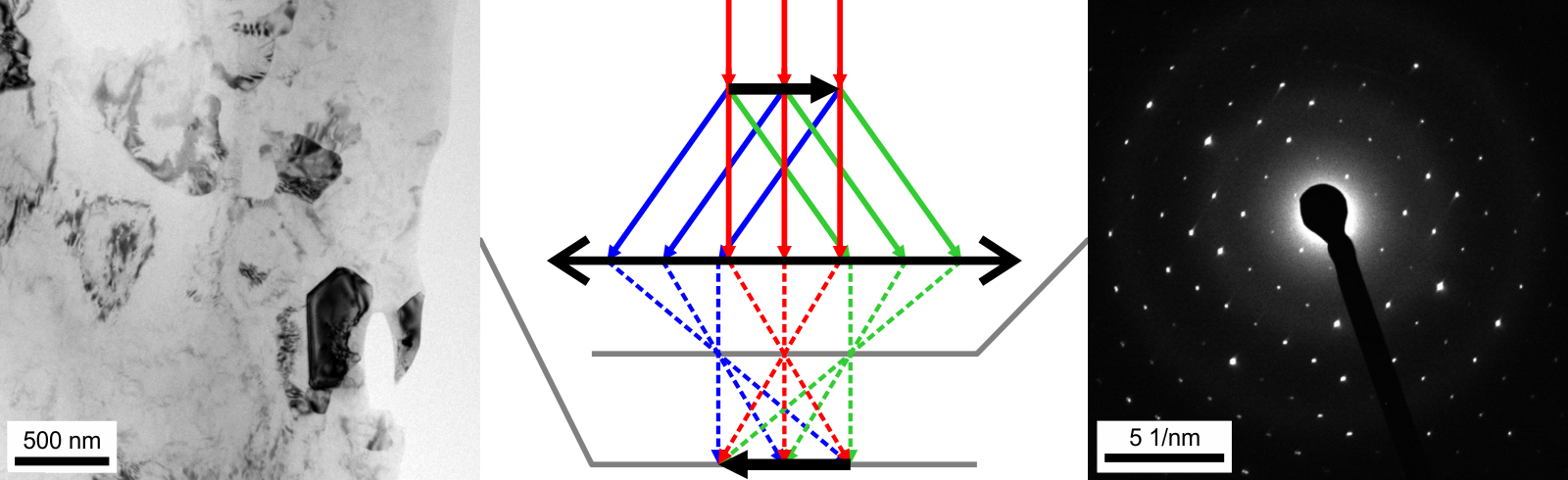 In TEM, the electron beam passes through a thin film of the material as illustrated in Figure 10. Before and after the sample the beam is manipulated by the electron optics
In TEM, the electron beam passes through a thin film of the material as illustrated in Figure 10. Before and after the sample the beam is manipulated by the electron opticswavefunction collapse
In various interpretations of quantum mechanics, wave function collapse, also called reduction of the state vector, occurs when a wave function—initially in a superposition of several eigenstates—reduces to a single eigenstate due to i ...
) according to the Copenhagen interpretation.
Selected area electron diffraction
The simplest diffraction technique in TEM is selected area electron diffraction (SAED) where the incident beam is wide and close to parallel.tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
 If the sample is tilted relative to the electron beam, different sets of crystallographic planes contribute to the pattern yielding different types of diffraction patterns, approximately different projections of the reciprocal lattice, see Figure 11.
If the sample is tilted relative to the electron beam, different sets of crystallographic planes contribute to the pattern yielding different types of diffraction patterns, approximately different projections of the reciprocal lattice, see Figure 11.
Polycrystalline pattern
 Diffraction patterns depend on whether the beam is diffracted by one
Diffraction patterns depend on whether the beam is diffracted by one single crystal
In materials science, a single crystal (or single-crystal solid or monocrystalline solid) is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no Grain boundary, grain bound ...
or by a number of differently oriented crystallites, for instance in a polycrystalline material. If there are many contributing crystallites, the diffraction image is a superposition of individual crystal patterns, see Figure 12. With a large number of grains this superposition yields diffraction spots of all possible reciprocal lattice vectors. This results in a pattern of concentric
In geometry, two or more objects are said to be ''concentric'' when they share the same center. Any pair of (possibly unalike) objects with well-defined centers can be concentric, including circles, spheres, regular polygons, regular polyh ...
rings as shown in Figure 12 and 13.
Multiple materials and double diffraction
In simple cases there is only one grain or one type of material in the area used for collecting a diffraction pattern. However, often there is more than one. If they are in different areas then the diffraction pattern will be a combination.
Bulk and surface superstructures
Many materials have relatively simple structures based upon small unit cell vectors (see also note). There are many others where the repeat is some larger multiple of the smaller unit cell (subcell) along one or more direction, for instance . which has larger dimensions in two directions. These superstructures[ can arise from many reasons:
# Larger unit cells due to electronic ordering which leads to small displacements of the atoms in the subcell. One example is antiferroelectricity ordering.
# Chemical ordering, that is different atom types at different locations of the subcell.
# Magnetic order of the spins. These may be in opposite directions on some atoms, leading to what is called antiferromagnetism.
In addition to those which occur in the bulk, superstructures can also occur at surfaces. When half the material is (nominally) removed to create a surface, some of the atoms will be under coordinated. To reduce their energy they can rearrange. Sometimes these rearrangements are relatively small; sometimes they are quite large. Similar to a bulk superstructure there will be additional, weaker diffraction spots. One example is for the silicon (111) surface, where there is a supercell which is seven times larger than the simple bulk cell in two directions.]
Aperiodic materials
In an aperiodic crystal the structure can no longer be simply described by three different vectors in real or reciprocal space. In general there is a substructure describable by three (e.g. ), similar to supercells above, but in addition there is some additional periodicity (one to three) which cannot be described as a multiple of the three; it is a genuine additional periodicity which is an irrational number
In mathematics, the irrational numbers are all the real numbers that are not rational numbers. That is, irrational numbers cannot be expressed as the ratio of two integers. When the ratio of lengths of two line segments is an irrational number, ...
relative to the subcell lattice.quasicrystals
A quasiperiodicity, quasiperiodic crystal, or quasicrystal, is a structure that is Order and disorder (physics), ordered but not Bravais lattice, periodic. A quasicrystalline pattern can continuously fill all available space, but it lacks trans ...
, which can be described similarly by a higher number of Miller indices in reciprocal space—but not by any translational symmetry in real space. An example of this is shown in Figure 15 for an Al–Cu–Fe–Cr decagonal quasicrystal grown by magnetron sputtering on a sodium chloride substrate and then lifted off by dissolving the substrate with water. In the pattern there are pentagons which are a characteristic of the aperiodic nature of these materials.
Diffuse scattering
 A further step beyond superstructures and aperiodic materials is what is called ''diffuse scattering'' in electron diffraction patterns due to disorder,
A further step beyond superstructures and aperiodic materials is what is called ''diffuse scattering'' in electron diffraction patterns due to disorder,probability distribution
In probability theory and statistics, a probability distribution is a Function (mathematics), function that gives the probabilities of occurrence of possible events for an Experiment (probability theory), experiment. It is a mathematical descri ...
for the distances between point defects or what type of substitutional atom there is, which leads to distinct three-dimensional intensity features in diffraction patterns. An example of this is for a Nb0.83CoSb sample, with the diffraction pattern shown in Figure 16. Because of the vacancies at the niobium sites, there is diffuse intensity with snake-like structure due to correlations of the distances between vacancies and also the relaxation of Co and Sb atoms around these vacancies.
Convergent beam electron diffraction
 In convergent beam electron diffraction (CBED),
In convergent beam electron diffraction (CBED), A CBED pattern consists of disks arranged similar to the spots in SAED. Intensity within the disks represents dynamical diffraction effects and symmetries of the sample structure, see Figure 7 and 18. Even though the zone axis and lattice parameter analysis based on disk positions does not significantly differ from SAED, the analysis of disks content is more complex and simulations based on dynamical diffraction theory is often required. As illustrated in Figure 18, the details within the disk change with sample thickness, as does the inelastic background. With appropriate analysis CBED patterns can be used for indexation of the crystal point group, space group identification, measurement of lattice parameters, thickness or strain.
A CBED pattern consists of disks arranged similar to the spots in SAED. Intensity within the disks represents dynamical diffraction effects and symmetries of the sample structure, see Figure 7 and 18. Even though the zone axis and lattice parameter analysis based on disk positions does not significantly differ from SAED, the analysis of disks content is more complex and simulations based on dynamical diffraction theory is often required. As illustrated in Figure 18, the details within the disk change with sample thickness, as does the inelastic background. With appropriate analysis CBED patterns can be used for indexation of the crystal point group, space group identification, measurement of lattice parameters, thickness or strain.
Precession electron diffraction
Precession electron diffraction (PED), invented by Roger Vincent and Paul Midgley in 1994, is a method to collect electron diffraction patterns in a transmission electron microscope
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a gr ...
(TEM). The technique involves rotating (precessing) a tilted incident electron beam around the central axis of the microscope, compensating for the tilt after the sample so a spot diffraction pattern is formed, similar to a SAED pattern. However, a PED pattern is an integration over a collection of diffraction conditions, see Figure 19. This integration produces a quasi-kinematical diffraction pattern that is more suitable as input into direct methods algorithms using electronscrystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
of the sample. Because it avoids many dynamical effects it can also be used to better identify crystallographic phases.
4D STEM
4D scanning transmission electron microscopy (4D STEM)EELS
Eels are ray-finned fish belonging to the order (biology), order Anguilliformes (), which consists of eight suborders, 20 Family (biology), families, 164 genus, genera, and about 1000 species. Eels undergo considerable development from the earl ...
, ND STEM (N- since the number of dimensions could be higher than 4), position resolved diffraction (PRD), spatial resolved diffractometry, momentum-resolved STEM, "nanobeam precision electron diffraction", scanning electron nano diffraction, nanobeam electron diffraction, or pixelated STEM. Most of these are the same, although there are instances such as momentum-resolved STEM where the emphasis can be very different.
Low-energy electron diffraction (LEED)
Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single-crystalline materials by bombardment with a collimated beam of low-energy electrons (30–200 eV).
Reflection high-energy electron diffraction (RHEED)
Reflection high energy electron diffraction (RHEED),technique
Technique or techniques may refer to:
Music
* The Techniques, a Jamaican rocksteady vocal group of the 1960s
* Technique (band), a British female synth pop band in the 1990s
* ''Technique'' (album), by New Order, 1989
* ''Techniques'' (album), by ...
used to characterize the surface of crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
materials by reflecting electrons off a surface. As illustrated for the Ewald sphere construction in Figure 22, it uses mainly the higher-order Laue zones which have a reflection component. An experimental diffraction pattern is shown in Figure 23 and shows both rings from the higher-order Laue zones and streaky spots.electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
. Transmission electron microscopy samples mainly the bulk of the sample, although in special cases it can provide surface information. Low-energy electron diffraction
Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single crystal, single-crystalline materials by bombardment with a collimated beam of low-energy electrons (30–200 eV) and observation o ...
(LEED) is also surface sensitive, and achieves surface sensitivity through the use of low energy electrons. The main uses of RHEED to date have been during thin film growth,
Gas electron diffraction
 Gas electron diffraction (GED) can be used to determine the
Gas electron diffraction (GED) can be used to determine the geometry
Geometry (; ) is a branch of mathematics concerned with properties of space such as the distance, shape, size, and relative position of figures. Geometry is, along with arithmetic, one of the oldest branches of mathematics. A mathematician w ...
of molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s in gases.
In a scanning electron microscope
In a scanning electron microscope
A scanning electron microscope (SEM) is a type of electron microscope that produces images of a sample by scanning the surface with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that ...
the region near the surface can be mapped using an electron beam that is scanned in a grid across the sample. A diffraction pattern can be recorded using electron backscatter diffraction (EBSD), as illustrated in Figure 25, captured with a camera inside the microscope. A depth from a few nanometers to a few microns, depending upon the electron energy used, is penetrated by the electrons, some of which are diffracted backwards and out of the sample. As result of combined inelastic and elastic scattering, typical features in an EBSD image are Kikuchi lines. Since the position of Kikuchi bands is highly sensitive to the crystal orientation, EBSD data can be used to determine the crystal orientation at particular locations of the sample. The data are processed by software yielding two-dimensional orientation maps.
Notes
References
Further reading
* . Contains extensive coverage of kinematical and other diffraction.
* Large coverage of many different areas of electron microscopy with large numbers of references.
* , often called the bible of electron microscopy.
* , a large coverage of topic related to dynamical diffraction and CBED
* . Very extensive coverage of modern dynamical diffraction.
* , a recent textbook with many images, stronger on experimental aspects.
* , an older source for experimental details, albeit hard to find.
{{Authority control
Applied and interdisciplinary physics
Crystallography
Diffraction
Electron
Electron microscopy
Materials science
Quantum mechanics
Scattering
 Building an electron microscope involves combining these elements, similar to an
Building an electron microscope involves combining these elements, similar to an  Around each reciprocal lattice point one has this shape function. How much intensity there will be in the diffraction pattern depends upon the intersection of the Ewald sphere, that is energy conservation, and the shape function around each reciprocal lattice point—see Figure 6, 20 and 22. The vector from a reciprocal lattice point to the Ewald sphere is called the excitation error .
For transmission electron diffraction the samples used are thin, so most of the shape function is along the direction of the electron beam. For both LEED and RHEED the shape function is mainly normal to the surface of the sample. In LEED this results in (a simplification) back-reflection of the electrons leading to spots, see Figure 20 and 21 later, whereas in RHEED the electrons reflect off the surface at a small angle and typically yield diffraction patterns with streaks, see Figure 22 and 23 later. By comparison, with both x-ray and neutron diffraction the scattering is significantly weaker, so typically requires much larger crystals, in which case the shape function shrinks to just around the reciprocal lattice points, leading to simpler Bragg's law diffraction.
For all cases, when the reciprocal lattice points are close to the Ewald sphere (the excitation error is small) the intensity tends to be higher; when they are far away it tends to be smaller. The set of diffraction spots at right angles to the direction of the incident beam are called the zero-order Laue zone (ZOLZ) spots, as shown in Figure 6. One can also have intensities further out from reciprocal lattice points which are in a higher layer. The first of these is called the first order Laue zone (FOLZ); the series is called by the generic name higher order Laue zone (HOLZ).
The result is that the electron wave after it has been diffracted can be written as an integral over different plane waves:that is a sum of plane waves going in different directions, each with a complex amplitude . (This is a three dimensional integral, which is often written as rather than .) For a crystalline sample these wavevectors have to be of the same magnitude for elastic scattering (no change in energy), and are related to the incident direction by (see Figure 6)
A diffraction pattern detects the intensitiesFor a crystal these will be near the reciprocal lattice points typically forming a two dimensional grid. Different samples and modes of diffraction give different results, as do different approximations for the amplitudes .
A typical electron diffraction pattern in TEM and LEED is a grid of high intensity spots (white) on a dark background, approximating a projection of the reciprocal lattice vectors, see Figure 1, 9, 10, 11, 14 and 21 later. There are also cases which will be mentioned later where diffraction patterns are not periodic, see Figure 15, have additional diffuse structure as in Figure 16, or have rings as in Figure 12, 13 and 24. With conical illumination as in CBED they can also be a grid of discs, see Figure 7, 9 and 18. RHEED is slightly different, see Figure 22, 23. If the excitation errors were zero for every reciprocal lattice vector, this grid would be at exactly the spacings of the reciprocal lattice vectors. This would be equivalent to a Bragg's law condition for all of them. In TEM the wavelength is small and this is close to correct, but not exact. In practice the deviation of the positions from a simple Bragg's law interpretation is often neglected, particularly if a column approximation is made (see below).
Around each reciprocal lattice point one has this shape function. How much intensity there will be in the diffraction pattern depends upon the intersection of the Ewald sphere, that is energy conservation, and the shape function around each reciprocal lattice point—see Figure 6, 20 and 22. The vector from a reciprocal lattice point to the Ewald sphere is called the excitation error .
For transmission electron diffraction the samples used are thin, so most of the shape function is along the direction of the electron beam. For both LEED and RHEED the shape function is mainly normal to the surface of the sample. In LEED this results in (a simplification) back-reflection of the electrons leading to spots, see Figure 20 and 21 later, whereas in RHEED the electrons reflect off the surface at a small angle and typically yield diffraction patterns with streaks, see Figure 22 and 23 later. By comparison, with both x-ray and neutron diffraction the scattering is significantly weaker, so typically requires much larger crystals, in which case the shape function shrinks to just around the reciprocal lattice points, leading to simpler Bragg's law diffraction.
For all cases, when the reciprocal lattice points are close to the Ewald sphere (the excitation error is small) the intensity tends to be higher; when they are far away it tends to be smaller. The set of diffraction spots at right angles to the direction of the incident beam are called the zero-order Laue zone (ZOLZ) spots, as shown in Figure 6. One can also have intensities further out from reciprocal lattice points which are in a higher layer. The first of these is called the first order Laue zone (FOLZ); the series is called by the generic name higher order Laue zone (HOLZ).
The result is that the electron wave after it has been diffracted can be written as an integral over different plane waves:that is a sum of plane waves going in different directions, each with a complex amplitude . (This is a three dimensional integral, which is often written as rather than .) For a crystalline sample these wavevectors have to be of the same magnitude for elastic scattering (no change in energy), and are related to the incident direction by (see Figure 6)
A diffraction pattern detects the intensitiesFor a crystal these will be near the reciprocal lattice points typically forming a two dimensional grid. Different samples and modes of diffraction give different results, as do different approximations for the amplitudes .
A typical electron diffraction pattern in TEM and LEED is a grid of high intensity spots (white) on a dark background, approximating a projection of the reciprocal lattice vectors, see Figure 1, 9, 10, 11, 14 and 21 later. There are also cases which will be mentioned later where diffraction patterns are not periodic, see Figure 15, have additional diffuse structure as in Figure 16, or have rings as in Figure 12, 13 and 24. With conical illumination as in CBED they can also be a grid of discs, see Figure 7, 9 and 18. RHEED is slightly different, see Figure 22, 23. If the excitation errors were zero for every reciprocal lattice vector, this grid would be at exactly the spacings of the reciprocal lattice vectors. This would be equivalent to a Bragg's law condition for all of them. In TEM the wavelength is small and this is close to correct, but not exact. In practice the deviation of the positions from a simple Bragg's law interpretation is often neglected, particularly if a column approximation is made (see below).
 The main components of current dynamical diffraction of electrons include:
* Taking into account the scattering back into the incident beam both from diffracted beams and between all others, not just single scattering from the incident beam to diffracted beams. This is important even for samples which are only a few atoms thick.
* Modelling at least semi-empirically the role of inelastic scattering by an imaginary component of the potential, also called an "optical potential". There is always inelastic scattering, and often it can have a major effect on both the background and sometimes the details, see Figure 7 and 18.
* Higher-order numerical approaches to calculate the intensities such as multislice, matrix methods which are called Bloch-wave approaches or muffin-tin approaches. With these diffraction spots which are not present in kinematical theory can be present, e.g.
* Contributions to the diffraction from elastic strain and
The main components of current dynamical diffraction of electrons include:
* Taking into account the scattering back into the incident beam both from diffracted beams and between all others, not just single scattering from the incident beam to diffracted beams. This is important even for samples which are only a few atoms thick.
* Modelling at least semi-empirically the role of inelastic scattering by an imaginary component of the potential, also called an "optical potential". There is always inelastic scattering, and often it can have a major effect on both the background and sometimes the details, see Figure 7 and 18.
* Higher-order numerical approaches to calculate the intensities such as multislice, matrix methods which are called Bloch-wave approaches or muffin-tin approaches. With these diffraction spots which are not present in kinematical theory can be present, e.g.
* Contributions to the diffraction from elastic strain and  Kikuchi lines come in pairs forming Kikuchi bands, and are indexed in terms of the crystallographic planes they are connected to, with the angular width of the band equal to the magnitude of the corresponding diffraction vector . The position of Kikuchi bands is fixed with respect to each other and the orientation of the sample, but not against the diffraction spots or the direction of the incident electron beam. As the crystal is tilted, the bands move on the diffraction pattern. Since the position of Kikuchi bands is quite sensitive to crystal orientation, they can be used to fine-tune a zone-axis orientation or determine crystal orientation. They can also be used for navigation when changing the orientation between zone axes connected by some band, an example of such a map produced by combining many local sets of experimental Kikuchi patterns is in Figure 8; Kikuchi maps are available for many materials.
Kikuchi lines come in pairs forming Kikuchi bands, and are indexed in terms of the crystallographic planes they are connected to, with the angular width of the band equal to the magnitude of the corresponding diffraction vector . The position of Kikuchi bands is fixed with respect to each other and the orientation of the sample, but not against the diffraction spots or the direction of the incident electron beam. As the crystal is tilted, the bands move on the diffraction pattern. Since the position of Kikuchi bands is quite sensitive to crystal orientation, they can be used to fine-tune a zone-axis orientation or determine crystal orientation. They can also be used for navigation when changing the orientation between zone axes connected by some band, an example of such a map produced by combining many local sets of experimental Kikuchi patterns is in Figure 8; Kikuchi maps are available for many materials.
 Electron diffraction in a TEM exploits controlled electron beams using electron optics. Different types of diffraction experiments, for instance Figure 9, provide information such as lattice constants, symmetries, and sometimes to solve an unknown
Electron diffraction in a TEM exploits controlled electron beams using electron optics. Different types of diffraction experiments, for instance Figure 9, provide information such as lattice constants, symmetries, and sometimes to solve an unknown  In TEM, the electron beam passes through a thin film of the material as illustrated in Figure 10. Before and after the sample the beam is manipulated by the electron optics including magnetic lenses, deflectors and apertures; these act on the electrons similar to how glass lenses focus and control light. Optical elements above the sample are used to control the incident beam which can range from a wide and parallel beam to one which is a converging cone and can be smaller than an atom, 0.1 nm. As it interacts with the sample, part of the beam is diffracted and part is transmitted without changing its direction. This occurs simultaneously as electrons are everywhere until they are detected (
In TEM, the electron beam passes through a thin film of the material as illustrated in Figure 10. Before and after the sample the beam is manipulated by the electron optics including magnetic lenses, deflectors and apertures; these act on the electrons similar to how glass lenses focus and control light. Optical elements above the sample are used to control the incident beam which can range from a wide and parallel beam to one which is a converging cone and can be smaller than an atom, 0.1 nm. As it interacts with the sample, part of the beam is diffracted and part is transmitted without changing its direction. This occurs simultaneously as electrons are everywhere until they are detected ( If the sample is tilted relative to the electron beam, different sets of crystallographic planes contribute to the pattern yielding different types of diffraction patterns, approximately different projections of the reciprocal lattice, see Figure 11. This can be used to determine the crystal orientation, which in turn can be used to set the orientation needed for a particular experiment. Furthermore, a series of diffraction patterns varying in tilt can be acquired and processed using a diffraction tomography approach. There are ways to combine this with direct methods algorithms using electrons and other methods such as charge flipping, or automated diffraction tomography to solve crystal structures.
If the sample is tilted relative to the electron beam, different sets of crystallographic planes contribute to the pattern yielding different types of diffraction patterns, approximately different projections of the reciprocal lattice, see Figure 11. This can be used to determine the crystal orientation, which in turn can be used to set the orientation needed for a particular experiment. Furthermore, a series of diffraction patterns varying in tilt can be acquired and processed using a diffraction tomography approach. There are ways to combine this with direct methods algorithms using electrons and other methods such as charge flipping, or automated diffraction tomography to solve crystal structures.
 Diffraction patterns depend on whether the beam is diffracted by one
Diffraction patterns depend on whether the beam is diffracted by one  A further step beyond superstructures and aperiodic materials is what is called ''diffuse scattering'' in electron diffraction patterns due to disorder, which is also known for x-ray or neutron scattering. This can occur from inelastic processes, for instance, in bulk silicon the atomic vibrations ( phonons) are more prevalent along specific directions, which leads to streaks in diffraction patterns. Sometimes it is due to arrangements of point defects. Completely disordered substitutional point defects lead to a general background which is called ''Laue monotonic scattering.'' Often there is a
A further step beyond superstructures and aperiodic materials is what is called ''diffuse scattering'' in electron diffraction patterns due to disorder, which is also known for x-ray or neutron scattering. This can occur from inelastic processes, for instance, in bulk silicon the atomic vibrations ( phonons) are more prevalent along specific directions, which leads to streaks in diffraction patterns. Sometimes it is due to arrangements of point defects. Completely disordered substitutional point defects lead to a general background which is called ''Laue monotonic scattering.'' Often there is a  In convergent beam electron diffraction (CBED), the incident electrons are normally focused in a converging cone-shaped beam with a crossover located at the sample, e.g. Figure 17, although other methods exist. Unlike the parallel beam, the convergent beam is able to carry information from the sample volume, not just a two-dimensional projection available in SAED. With convergent beam there is also no need for the selected area aperture, as it is inherently site-selective since the beam crossover is positioned at the object plane where the sample is located.
In convergent beam electron diffraction (CBED), the incident electrons are normally focused in a converging cone-shaped beam with a crossover located at the sample, e.g. Figure 17, although other methods exist. Unlike the parallel beam, the convergent beam is able to carry information from the sample volume, not just a two-dimensional projection available in SAED. With convergent beam there is also no need for the selected area aperture, as it is inherently site-selective since the beam crossover is positioned at the object plane where the sample is located.
 A CBED pattern consists of disks arranged similar to the spots in SAED. Intensity within the disks represents dynamical diffraction effects and symmetries of the sample structure, see Figure 7 and 18. Even though the zone axis and lattice parameter analysis based on disk positions does not significantly differ from SAED, the analysis of disks content is more complex and simulations based on dynamical diffraction theory is often required. As illustrated in Figure 18, the details within the disk change with sample thickness, as does the inelastic background. With appropriate analysis CBED patterns can be used for indexation of the crystal point group, space group identification, measurement of lattice parameters, thickness or strain.
The disk diameter can be controlled using the microscope optics and apertures. The larger is the angle, the broader the disks are with more features. If the angle is increased to significantly, the disks begin to overlap. This is avoided in large angle convergent electron beam diffraction (LACBED) where the sample is moved upwards or downwards. There are applications, however, where the overlapping disks are beneficial, for instance with a ronchigram. It is a CBED pattern, often but not always of an amorphous material, with many intentionally overlapping disks providing information about the optical aberrations of the electron optical system.
A CBED pattern consists of disks arranged similar to the spots in SAED. Intensity within the disks represents dynamical diffraction effects and symmetries of the sample structure, see Figure 7 and 18. Even though the zone axis and lattice parameter analysis based on disk positions does not significantly differ from SAED, the analysis of disks content is more complex and simulations based on dynamical diffraction theory is often required. As illustrated in Figure 18, the details within the disk change with sample thickness, as does the inelastic background. With appropriate analysis CBED patterns can be used for indexation of the crystal point group, space group identification, measurement of lattice parameters, thickness or strain.
The disk diameter can be controlled using the microscope optics and apertures. The larger is the angle, the broader the disks are with more features. If the angle is increased to significantly, the disks begin to overlap. This is avoided in large angle convergent electron beam diffraction (LACBED) where the sample is moved upwards or downwards. There are applications, however, where the overlapping disks are beneficial, for instance with a ronchigram. It is a CBED pattern, often but not always of an amorphous material, with many intentionally overlapping disks providing information about the optical aberrations of the electron optical system.
 Gas electron diffraction (GED) can be used to determine the
Gas electron diffraction (GED) can be used to determine the