Dichloro(1,3-bis(diphenylphosphino)propane)nickel on:
[Wikipedia]
[Google]
[Amazon]
Dichloro ,3-bis(diphenylphosphino)propaneickel a
coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
with the formula NiCl2(dppp); where dppp is the diphosphine
Diphosphane, or diphosphine, is an inorganic compound with the chemical formula . This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air.
Properties, ...
1,3-bis(diphenylphosphino)propane
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula PhP(CH)PPh. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is ...
. It is used as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
in organic synthesis. The compound is a bright orange-red crystalline powder.
Structure and properties
While the electronic and solid-state-supported structure of the chloride congener is not known (due to low solubility in common analytical solvents), several studies have been carried out on the bromo and iodo derivatives. The complexes display a temperature-dependent interconversion between square-planar and tetrahedral geometries (diamagnetic and paramagnetic) in polar organic solvents (Keq between 1-3.68, depending on the solvent and temperature). In contrast, dichloro(1,2-bis(diphenylphosphino)ethane)nickel adopts a static square-planar (diamagnetic) structure in solution.Preparation
NiCl2(dppp) is prepared by combining equal molar portions ofnickel(II) chloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for che ...
hexahydrate with 1,3-bis(diphenylphosphino)propane
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula PhP(CH)PPh. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is ...
in 2-propanol.
:Ni(H2O)6Cl2 + dppp → NiCl2(dppp) + 6 H2O
Reactions
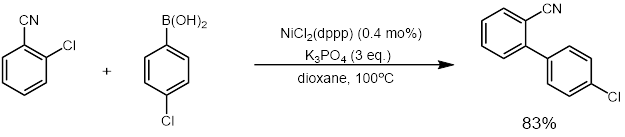
NiCl2(dppp) in an effective catalyst forcoupling reaction
In organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Such reactions often require the aid of a metal catalyst. In one important reaction type, a main group organometallic compound o ...
s such as the Kumada coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically ...
and Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
s (example below). It also catalyzes other reactions that convert enol ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers incl ...
s, dithioacetal
In organosulfur chemistry, thioacetals are the sulfur ('' thio-'') analogues of acetals (). There are two classes: the less-common monothioacetals, with the formula , and the dithioacetals, with the formula (symmetric dithioacetals) or (asy ...
s, and vinyl sulfides to olefins
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of P ...
.

References
{{Reflist, 1 Nickel complexes Phosphine complexes Chloro complexes