Crabtree's Catalyst on:
[Wikipedia]
[Google]
[Amazon]
Crabtree's catalyst is an organoiridium compound with the formula C8H12Ir P(C6H11)3pyridine">C5H5N">Tricyclohexylphosphine">P(C6H11)3pyridine">C5H5N/nowiki>PF6. It is a homogeneous catalyst for

 Crabtree's catalyst is used in
Crabtree's catalyst is used in

hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
and hydrogen-transfer reactions, developed by Robert H. Crabtree. This air stable orange solid is commercially available and known for its directed hydrogenation to give trans stereoselectivity with respective of directing group.
Structure and synthesis
The cation has asquare planar molecular geometry
In chemistry, the square planar molecular geometry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the co ...
, as expected for a d8 complex. It is prepared from cyclooctadiene iridium chloride dimer.
Reactivity
Crabtree’s catalyst is effective for the hydrogenations of mono-, di-, tri-, and tetra-substituted substrates. Whereas Wilkinson’s catalyst and the Schrock–Osborn catalyst do not catalyze the hydrogenation of a tetrasubstituted olefin, Crabtree’s catalyst does so to at high turnover frequencies (table). : The catalyst is reactive at room temperature. The reaction is robust without drying solvents or meticulous deoxygenation of the hydrogen. The catalyst is tolerant of weakly basic functional groups such as ester, but not alcohols (see below) or amines. The catalyst is sensitive to proton-bearing impurities. The catalyst becomes irreversibly deactivated after about ten minutes at room temperature, signaled by appearance of yellow color. One deactivation process involves formation of hydride-bridged dimers. As a consequence, Crabtree's Catalyst is usually used in very low catalyst loading.
Other catalytic functions: isotope exchange and isomerization
Besides hydrogenation, the catalyst catalyzes the isomerization and hydroboration of alkenes. Crabtree's catalyst is used in
Crabtree's catalyst is used in isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
exchange reactions. More specifically, it catalyzes the direct exchange of a hydrogen atom with its isotopes deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
and tritium
Tritium () or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with a half-life of ~12.33 years. The tritium nucleus (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the ...
, without the use of an intermediate. It has been shown that isotope exchange with Crabtree’s catalyst is highly regioselective.
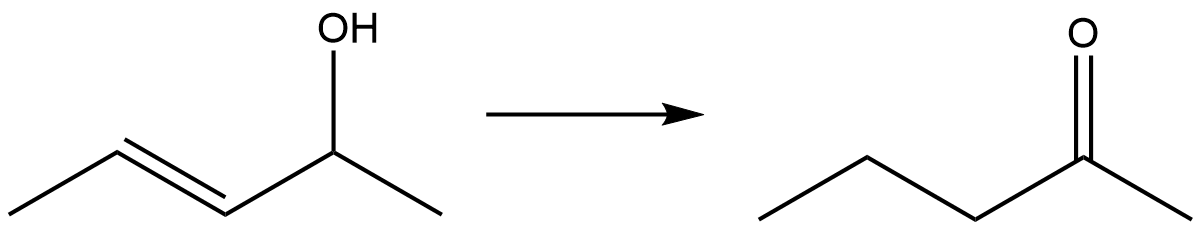
Influence of directing functional groups
Thehydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
of a terpen-4-ol demonstrates the ability of compounds with directing groups (the –OH group) to undergo diastereoselective hydrogenation. With palladium on carbon in ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
the product distribution is 20:80 favoring the ''cis'' isomer (2B in Scheme 1). The polar side (with the hydroxyl group) interacts with the solvent. This is due to slight haptophilicity, an effect in which a functional group binds to the surface of a heterogeneous catalyst and directs the reaction. In cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
as solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
, the distribution changes to 53:47 because haptophilicity is no long present (there is no directing group on cyclohexane). The distribution changes completely in favor of the ''trans'' isomer 2A when Crabtree's catalyst is used in dichloromethane
Dichloromethane (DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with ...
. This selectivity is both predictable and practically useful. Carbonyl groups are also known to direct the hydrogenation by the Crabtree catalyst to be highly regioselective.
:
The directing effect that causes the stereoselectivity of hydrogenation of terpen-4-ol with Crabtree’s catalyst is shown below.

History
Crabtree and graduate student George Morris discovered this catalyst in the 1970s while working on iridium analogues ofWilkinson's
Wilko.com Limited (trading as Wilko) is a British Variety store, variety retailer. It was founded as Wilkinson by James Kemsey Wilkinson and Mary Cooper in 1930 as a hardware retailer, opening its first store in Leicester.
In 1972, Tony Wil ...
rhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
-based catalyst at the Institut de Chimie des Substances Naturelles
The Institut de Chimie des Substances Naturelles ("Institute for the chemistry of natural substances"), or ICSN, is part of the Centre national de la recherche scientifique, France's most prominent public research organization.
Located at Gif-s ...
at Gif-sur-Yvette
Gif-sur-Yvette (, "Gif-on- Yvette") is a commune in southwestern Île-de-France, France. It is located in the Vallée de Chevreuse, from the centre of Paris (at Notre-Dame), in the Essonne department on the departmental border with Yvelin ...
, near Paris.
Previous hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
catalysts included Wilkinson’s catalyst and a cationic rhodium(I) complex with two phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
groups developed by Osborn and Schrock. These catalysts accomplished hydrogenation through displacement; after hydrogen addition across the metal, a solvent or a phosphine group dissociated from the rhodium metal so the olefin to be hydrogenated could gain access to the active site. This displacement occurs quickly for rhodium complexes but occurs barely at all for iridium complexes. Because of this, research at the time focused on rhodium compounds instead of compounds involving transition metals of the third row, like iridium. Wilkinson, Osborn, and Schrock also only used coordinating solvents.
Crabtree noted that the ligand dissociation step does not occur in heterogeneous catalysis
Heterogeneous catalysis is catalysis where the Phase (matter), phase of catalysts differs from that of the reagents or product (chemistry), products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exis ...
, and so posited that this step was limiting in homogeneous systems. They sought catalysts with "irreversibly created active sites in a noncoordinating solvent." This led to the development of the Crabtree catalyst, and use of the solvent CH2Cl2.
References
{{Iridium compounds Homogeneous catalysis Cyclooctadiene complexes Hydrogenation catalysts Organoiridium compounds Phosphine complexes Hexafluorophosphates