Conjugated system on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 Conjugation is possible by means of alternating single and
Conjugation is possible by means of alternating single and  A common model for the treatment of conjugated molecules is a composite valence bond / Hückel molecular orbital theory (VB/HMOT) treatment, in which the σ framework of the molecule is separated from the π system (or systems) of the molecule (''see the article on the sigma-pi and equivalent-orbital models for this model and an alternative treatment''). Although σ bonding can be treated using a delocalized approach as well, it is generally the π bonding that is being considered when delocalized bonding is invoked in the context of simple organic molecules.
''Sigma (σ) framework'': The σ framework is described by a strictly localized bonding scheme and consists of σ bonds formed from the interactions between sp3-, sp2-, and sp- hybridized atomic orbitals on the main group elements (and 1s atomic orbitals on hydrogen), together with localized lone pairs derived from filled, nonbonding hybrid orbitals. The interaction that results in σ bonding takes the form of head-to-head overlap of the larger lobe of each hybrid orbital (or the single spherical lobe of a hydrogen 1s orbital). Each atomic orbital contributes one electron when the orbitals overlap pairwise to form two-electron σ bonds, or two electrons when the orbital constitutes a lone pair. These localized orbitals (bonding and non-bonding) are all located in the plane of the molecule, with σ bonds mainly localized between nuclei along the internuclear axis.
''Pi (π) system or systems'': Orthogonal to the σ framework described above, π bonding occurs above and below the plane of the molecule where σ bonding takes place. The π system(s) of the molecule are formed by the interaction of unhybridized p atomic orbitals on atoms employing sp2- and sp-hybridization. The interaction that results in π bonding takes place between p orbitals that are adjacent by virtue of a σ bond joining the atoms and takes the form of side-to-side overlap of the two equally large lobes that make up each p orbital. Atoms that are sp3-hybridized do not have an unhybridized p orbital available for participation in π bonding and their presence necessarily terminates a π system or separates two π systems. A basis p orbital that takes part in a π system can contribute one electron (which corresponds to half of a formal "double bond"), two electrons (which corresponds to a delocalized "lone pair"), or zero electrons (which corresponds to a formally "empty" orbital). Bonding for π systems formed from the overlap of more than two p orbitals is handled using the Hückel approach to obtain a zeroth order (qualitative) approximation of the π symmetry molecular orbitals that result from delocalized π bonding.
A common model for the treatment of conjugated molecules is a composite valence bond / Hückel molecular orbital theory (VB/HMOT) treatment, in which the σ framework of the molecule is separated from the π system (or systems) of the molecule (''see the article on the sigma-pi and equivalent-orbital models for this model and an alternative treatment''). Although σ bonding can be treated using a delocalized approach as well, it is generally the π bonding that is being considered when delocalized bonding is invoked in the context of simple organic molecules.
''Sigma (σ) framework'': The σ framework is described by a strictly localized bonding scheme and consists of σ bonds formed from the interactions between sp3-, sp2-, and sp- hybridized atomic orbitals on the main group elements (and 1s atomic orbitals on hydrogen), together with localized lone pairs derived from filled, nonbonding hybrid orbitals. The interaction that results in σ bonding takes the form of head-to-head overlap of the larger lobe of each hybrid orbital (or the single spherical lobe of a hydrogen 1s orbital). Each atomic orbital contributes one electron when the orbitals overlap pairwise to form two-electron σ bonds, or two electrons when the orbital constitutes a lone pair. These localized orbitals (bonding and non-bonding) are all located in the plane of the molecule, with σ bonds mainly localized between nuclei along the internuclear axis.
''Pi (π) system or systems'': Orthogonal to the σ framework described above, π bonding occurs above and below the plane of the molecule where σ bonding takes place. The π system(s) of the molecule are formed by the interaction of unhybridized p atomic orbitals on atoms employing sp2- and sp-hybridization. The interaction that results in π bonding takes place between p orbitals that are adjacent by virtue of a σ bond joining the atoms and takes the form of side-to-side overlap of the two equally large lobes that make up each p orbital. Atoms that are sp3-hybridized do not have an unhybridized p orbital available for participation in π bonding and their presence necessarily terminates a π system or separates two π systems. A basis p orbital that takes part in a π system can contribute one electron (which corresponds to half of a formal "double bond"), two electrons (which corresponds to a delocalized "lone pair"), or zero electrons (which corresponds to a formally "empty" orbital). Bonding for π systems formed from the overlap of more than two p orbitals is handled using the Hückel approach to obtain a zeroth order (qualitative) approximation of the π symmetry molecular orbitals that result from delocalized π bonding.
 This simple model for chemical bonding is successful for the description of most normal-valence molecules consisting of only s- and p-block elements, although systems that involve electron-deficient bonding, including nonclassical carbocations, lithium and boron clusters, and hypervalent centers require significant modifications in which σ bonds are also allowed to delocalize and are perhaps better treated with canonical molecular orbitals that are delocalized over the entire molecule. Likewise, d- and f-block organometallics are also inadequately described by this simple model. Bonds in strained small rings (such as cyclopropane or epoxide) are not well-described by strict σ/π separation, as bonding between atoms in the ring consists of " bent bonds" or "banana bonds" that are bowed outward and are intermediate in nature between σ and π bonds. Nevertheless, organic chemists frequently use the language of this model to rationalize the structure and reactivity of typical organic compounds.
Electrons in conjugated π systems are shared by all adjacent sp2- and sp-hybridized atoms that contribute overlapping, parallel p atomic orbitals. As such, the atoms and π-electrons involved behave as one large bonded system. These systems are often referred to n''-center ''k-''electron π-bonds,' compactly denoted by the symbol Π, to emphasize this behavior. For example, the delocalized π electrons in acetate anion and benzene are said to be involved in Π and Π systems, respectively (''see the article on three-center four-electron bonding''). Generally speaking, these multi-center bonds correspond to the occupation of several molecular orbitals (MOs) with varying degrees of bonding or non-bonding character (filling of orbitals with antibonding character is uncommon). Each one is occupied by one or two electrons in accordance with the
This simple model for chemical bonding is successful for the description of most normal-valence molecules consisting of only s- and p-block elements, although systems that involve electron-deficient bonding, including nonclassical carbocations, lithium and boron clusters, and hypervalent centers require significant modifications in which σ bonds are also allowed to delocalize and are perhaps better treated with canonical molecular orbitals that are delocalized over the entire molecule. Likewise, d- and f-block organometallics are also inadequately described by this simple model. Bonds in strained small rings (such as cyclopropane or epoxide) are not well-described by strict σ/π separation, as bonding between atoms in the ring consists of " bent bonds" or "banana bonds" that are bowed outward and are intermediate in nature between σ and π bonds. Nevertheless, organic chemists frequently use the language of this model to rationalize the structure and reactivity of typical organic compounds.
Electrons in conjugated π systems are shared by all adjacent sp2- and sp-hybridized atoms that contribute overlapping, parallel p atomic orbitals. As such, the atoms and π-electrons involved behave as one large bonded system. These systems are often referred to n''-center ''k-''electron π-bonds,' compactly denoted by the symbol Π, to emphasize this behavior. For example, the delocalized π electrons in acetate anion and benzene are said to be involved in Π and Π systems, respectively (''see the article on three-center four-electron bonding''). Generally speaking, these multi-center bonds correspond to the occupation of several molecular orbitals (MOs) with varying degrees of bonding or non-bonding character (filling of orbitals with antibonding character is uncommon). Each one is occupied by one or two electrons in accordance with the
 There are also other types of interactions that generalize the idea of interacting p orbitals in a conjugated system. The concept of ''hyperconjugation'' holds that certain σ bonds can also delocalize into a low-lying unoccupied orbital of a π system or an unoccupied p orbital. Hyperconjugation is commonly invoked to explain the stability of alkyl substituted radicals and carbocations. Hyperconjugation is less important for species in which all atoms satisfy the octet rule, but a recent computational study supports hyperconjugation as the origin of the increased stability of alkenes with a higher degree of substitution ( Zaitsev's rule).
''Homoconjugation'' is an overlap of two π-systems separated by a non-conjugating group, such as CH2. Unambiguous examples are comparatively rare in neutral systems, due to a comparatively minor energetic benefit that is easily overridden by a variety of other factors; however, they are common in cationic systems in which a large energetic benefit can be derived from delocalization of positive charge (''see the article on homoaromaticity for details.''). Neutral systems generally require constrained geometries favoring interaction to produce significant degrees of homoconjugation. In the example below, the carbonyl stretching frequencies of the IR spectra of the respective compounds demonstrate homoconjugation, or lack thereof, in the neutral ground state molecules.
Due to the partial π character of formally σ bonds in a cyclopropane ring, evidence for transmission of "conjugation" through cyclopropanes has also been obtained.
Two appropriately aligned π systems whose ends meet at right angles can engage in spiroconjugation or in homoconjugation across the spiro atom.
Vinylogy is the extension of a functional group through a conjugated organic bonding system, which transmits electronic effects.
There are also other types of interactions that generalize the idea of interacting p orbitals in a conjugated system. The concept of ''hyperconjugation'' holds that certain σ bonds can also delocalize into a low-lying unoccupied orbital of a π system or an unoccupied p orbital. Hyperconjugation is commonly invoked to explain the stability of alkyl substituted radicals and carbocations. Hyperconjugation is less important for species in which all atoms satisfy the octet rule, but a recent computational study supports hyperconjugation as the origin of the increased stability of alkenes with a higher degree of substitution ( Zaitsev's rule).
''Homoconjugation'' is an overlap of two π-systems separated by a non-conjugating group, such as CH2. Unambiguous examples are comparatively rare in neutral systems, due to a comparatively minor energetic benefit that is easily overridden by a variety of other factors; however, they are common in cationic systems in which a large energetic benefit can be derived from delocalization of positive charge (''see the article on homoaromaticity for details.''). Neutral systems generally require constrained geometries favoring interaction to produce significant degrees of homoconjugation. In the example below, the carbonyl stretching frequencies of the IR spectra of the respective compounds demonstrate homoconjugation, or lack thereof, in the neutral ground state molecules.
Due to the partial π character of formally σ bonds in a cyclopropane ring, evidence for transmission of "conjugation" through cyclopropanes has also been obtained.
Two appropriately aligned π systems whose ends meet at right angles can engage in spiroconjugation or in homoconjugation across the spiro atom.
Vinylogy is the extension of a functional group through a conjugated organic bonding system, which transmits electronic effects.

 Not all compounds with alternating double and single bonds are aromatic.
Not all compounds with alternating double and single bonds are aromatic.

 Conjugated chromophores are found in many
Conjugated chromophores are found in many  Conjugated polymer
Conjugated polymer
physical organic chemistry
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and chemical reaction, reactivity, in particular, applying experimental to ...
, a conjugated system is a system of connected p-orbitals with delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
s in a molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
, which in general lowers the overall energy of the molecule and increases stability
Stability may refer to:
Mathematics
*Stability theory, the study of the stability of solutions to differential equations and dynamical systems
** Asymptotic stability
** Exponential stability
** Linear stability
**Lyapunov stability
** Marginal s ...
. It is conventionally represented as having alternating single and multiple bonds. Lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear
In mathematics, the term ''linear'' is used in two distinct senses for two different properties:
* linearity of a '' function'' (or '' mapping'');
* linearity of a '' polynomial''.
An example of a linear function is the function defined by f(x) ...
or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.
Conjugation is the overlap of one p-orbital with another across an adjacent σ bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diatomi ...
(in transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s, d-orbitals can be involved).
A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
across all the adjacent aligned p-orbitals.
The π electrons do not belong to a single bond or atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
, but rather to a group of atoms.
Molecules containing conjugated systems of orbitals and electrons are called conjugated molecules, which have overlapping p orbitals on three or more atoms. Some simple organic conjugated molecules are 1,3-butadiene, benzene, and allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
carbocations. The largest conjugated systems are found in graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
, graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, conductive polymer
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The main advantage of conductive polymers ...
s and carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''S ...
s.
Chemical bonding in conjugated systems
 Conjugation is possible by means of alternating single and
Conjugation is possible by means of alternating single and double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s in which each atom supplies a p orbital perpendicular to the plane of the molecule. However, that is not the only way for conjugation to take place. As long as each contiguous atom in a chain has an available p orbital, the system can be considered conjugated. For example, furan is a five-membered ring with two alternating double bonds flanking an oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. The oxygen has two lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s, one of which occupies a p orbital perpendicular to the ring on that position, thereby maintaining the conjugation of that five-membered ring by overlap with the perpendicular p orbital on each of the adjacent carbon atoms. The other lone pair remains in plane and does not participate in conjugation.
In general, any sp2 or sp-hybridized carbon or heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
, including ones bearing an empty orbital or lone pair orbital, can participate in conjugated systems. However lone pairs do not always participate in a conjugated system. For example, in pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
, the nitrogen atom already participates in the conjugated system through a formal double bond with an adjacent carbon, so the lone pair remains in the plane of the ring in an sp2 hybrid orbital and does not participate in the conjugation. A requirement for conjugation is orbital overlap. Thus, the conjugated system must be planar (or nearly so). As a consequence, lone pairs which do participate in conjugated systems will occupy orbitals of pure p character instead of sp''n'' hybrid orbitals typical for nonconjugated lone pairs.
 A common model for the treatment of conjugated molecules is a composite valence bond / Hückel molecular orbital theory (VB/HMOT) treatment, in which the σ framework of the molecule is separated from the π system (or systems) of the molecule (''see the article on the sigma-pi and equivalent-orbital models for this model and an alternative treatment''). Although σ bonding can be treated using a delocalized approach as well, it is generally the π bonding that is being considered when delocalized bonding is invoked in the context of simple organic molecules.
''Sigma (σ) framework'': The σ framework is described by a strictly localized bonding scheme and consists of σ bonds formed from the interactions between sp3-, sp2-, and sp- hybridized atomic orbitals on the main group elements (and 1s atomic orbitals on hydrogen), together with localized lone pairs derived from filled, nonbonding hybrid orbitals. The interaction that results in σ bonding takes the form of head-to-head overlap of the larger lobe of each hybrid orbital (or the single spherical lobe of a hydrogen 1s orbital). Each atomic orbital contributes one electron when the orbitals overlap pairwise to form two-electron σ bonds, or two electrons when the orbital constitutes a lone pair. These localized orbitals (bonding and non-bonding) are all located in the plane of the molecule, with σ bonds mainly localized between nuclei along the internuclear axis.
''Pi (π) system or systems'': Orthogonal to the σ framework described above, π bonding occurs above and below the plane of the molecule where σ bonding takes place. The π system(s) of the molecule are formed by the interaction of unhybridized p atomic orbitals on atoms employing sp2- and sp-hybridization. The interaction that results in π bonding takes place between p orbitals that are adjacent by virtue of a σ bond joining the atoms and takes the form of side-to-side overlap of the two equally large lobes that make up each p orbital. Atoms that are sp3-hybridized do not have an unhybridized p orbital available for participation in π bonding and their presence necessarily terminates a π system or separates two π systems. A basis p orbital that takes part in a π system can contribute one electron (which corresponds to half of a formal "double bond"), two electrons (which corresponds to a delocalized "lone pair"), or zero electrons (which corresponds to a formally "empty" orbital). Bonding for π systems formed from the overlap of more than two p orbitals is handled using the Hückel approach to obtain a zeroth order (qualitative) approximation of the π symmetry molecular orbitals that result from delocalized π bonding.
A common model for the treatment of conjugated molecules is a composite valence bond / Hückel molecular orbital theory (VB/HMOT) treatment, in which the σ framework of the molecule is separated from the π system (or systems) of the molecule (''see the article on the sigma-pi and equivalent-orbital models for this model and an alternative treatment''). Although σ bonding can be treated using a delocalized approach as well, it is generally the π bonding that is being considered when delocalized bonding is invoked in the context of simple organic molecules.
''Sigma (σ) framework'': The σ framework is described by a strictly localized bonding scheme and consists of σ bonds formed from the interactions between sp3-, sp2-, and sp- hybridized atomic orbitals on the main group elements (and 1s atomic orbitals on hydrogen), together with localized lone pairs derived from filled, nonbonding hybrid orbitals. The interaction that results in σ bonding takes the form of head-to-head overlap of the larger lobe of each hybrid orbital (or the single spherical lobe of a hydrogen 1s orbital). Each atomic orbital contributes one electron when the orbitals overlap pairwise to form two-electron σ bonds, or two electrons when the orbital constitutes a lone pair. These localized orbitals (bonding and non-bonding) are all located in the plane of the molecule, with σ bonds mainly localized between nuclei along the internuclear axis.
''Pi (π) system or systems'': Orthogonal to the σ framework described above, π bonding occurs above and below the plane of the molecule where σ bonding takes place. The π system(s) of the molecule are formed by the interaction of unhybridized p atomic orbitals on atoms employing sp2- and sp-hybridization. The interaction that results in π bonding takes place between p orbitals that are adjacent by virtue of a σ bond joining the atoms and takes the form of side-to-side overlap of the two equally large lobes that make up each p orbital. Atoms that are sp3-hybridized do not have an unhybridized p orbital available for participation in π bonding and their presence necessarily terminates a π system or separates two π systems. A basis p orbital that takes part in a π system can contribute one electron (which corresponds to half of a formal "double bond"), two electrons (which corresponds to a delocalized "lone pair"), or zero electrons (which corresponds to a formally "empty" orbital). Bonding for π systems formed from the overlap of more than two p orbitals is handled using the Hückel approach to obtain a zeroth order (qualitative) approximation of the π symmetry molecular orbitals that result from delocalized π bonding.
 This simple model for chemical bonding is successful for the description of most normal-valence molecules consisting of only s- and p-block elements, although systems that involve electron-deficient bonding, including nonclassical carbocations, lithium and boron clusters, and hypervalent centers require significant modifications in which σ bonds are also allowed to delocalize and are perhaps better treated with canonical molecular orbitals that are delocalized over the entire molecule. Likewise, d- and f-block organometallics are also inadequately described by this simple model. Bonds in strained small rings (such as cyclopropane or epoxide) are not well-described by strict σ/π separation, as bonding between atoms in the ring consists of " bent bonds" or "banana bonds" that are bowed outward and are intermediate in nature between σ and π bonds. Nevertheless, organic chemists frequently use the language of this model to rationalize the structure and reactivity of typical organic compounds.
Electrons in conjugated π systems are shared by all adjacent sp2- and sp-hybridized atoms that contribute overlapping, parallel p atomic orbitals. As such, the atoms and π-electrons involved behave as one large bonded system. These systems are often referred to n''-center ''k-''electron π-bonds,' compactly denoted by the symbol Π, to emphasize this behavior. For example, the delocalized π electrons in acetate anion and benzene are said to be involved in Π and Π systems, respectively (''see the article on three-center four-electron bonding''). Generally speaking, these multi-center bonds correspond to the occupation of several molecular orbitals (MOs) with varying degrees of bonding or non-bonding character (filling of orbitals with antibonding character is uncommon). Each one is occupied by one or two electrons in accordance with the
This simple model for chemical bonding is successful for the description of most normal-valence molecules consisting of only s- and p-block elements, although systems that involve electron-deficient bonding, including nonclassical carbocations, lithium and boron clusters, and hypervalent centers require significant modifications in which σ bonds are also allowed to delocalize and are perhaps better treated with canonical molecular orbitals that are delocalized over the entire molecule. Likewise, d- and f-block organometallics are also inadequately described by this simple model. Bonds in strained small rings (such as cyclopropane or epoxide) are not well-described by strict σ/π separation, as bonding between atoms in the ring consists of " bent bonds" or "banana bonds" that are bowed outward and are intermediate in nature between σ and π bonds. Nevertheless, organic chemists frequently use the language of this model to rationalize the structure and reactivity of typical organic compounds.
Electrons in conjugated π systems are shared by all adjacent sp2- and sp-hybridized atoms that contribute overlapping, parallel p atomic orbitals. As such, the atoms and π-electrons involved behave as one large bonded system. These systems are often referred to n''-center ''k-''electron π-bonds,' compactly denoted by the symbol Π, to emphasize this behavior. For example, the delocalized π electrons in acetate anion and benzene are said to be involved in Π and Π systems, respectively (''see the article on three-center four-electron bonding''). Generally speaking, these multi-center bonds correspond to the occupation of several molecular orbitals (MOs) with varying degrees of bonding or non-bonding character (filling of orbitals with antibonding character is uncommon). Each one is occupied by one or two electrons in accordance with the Aufbau principle
In atomic physics and quantum chemistry, the Aufbau principle (, from ), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill Electron shell#Subshells, subshells of the lowest available energy, the ...
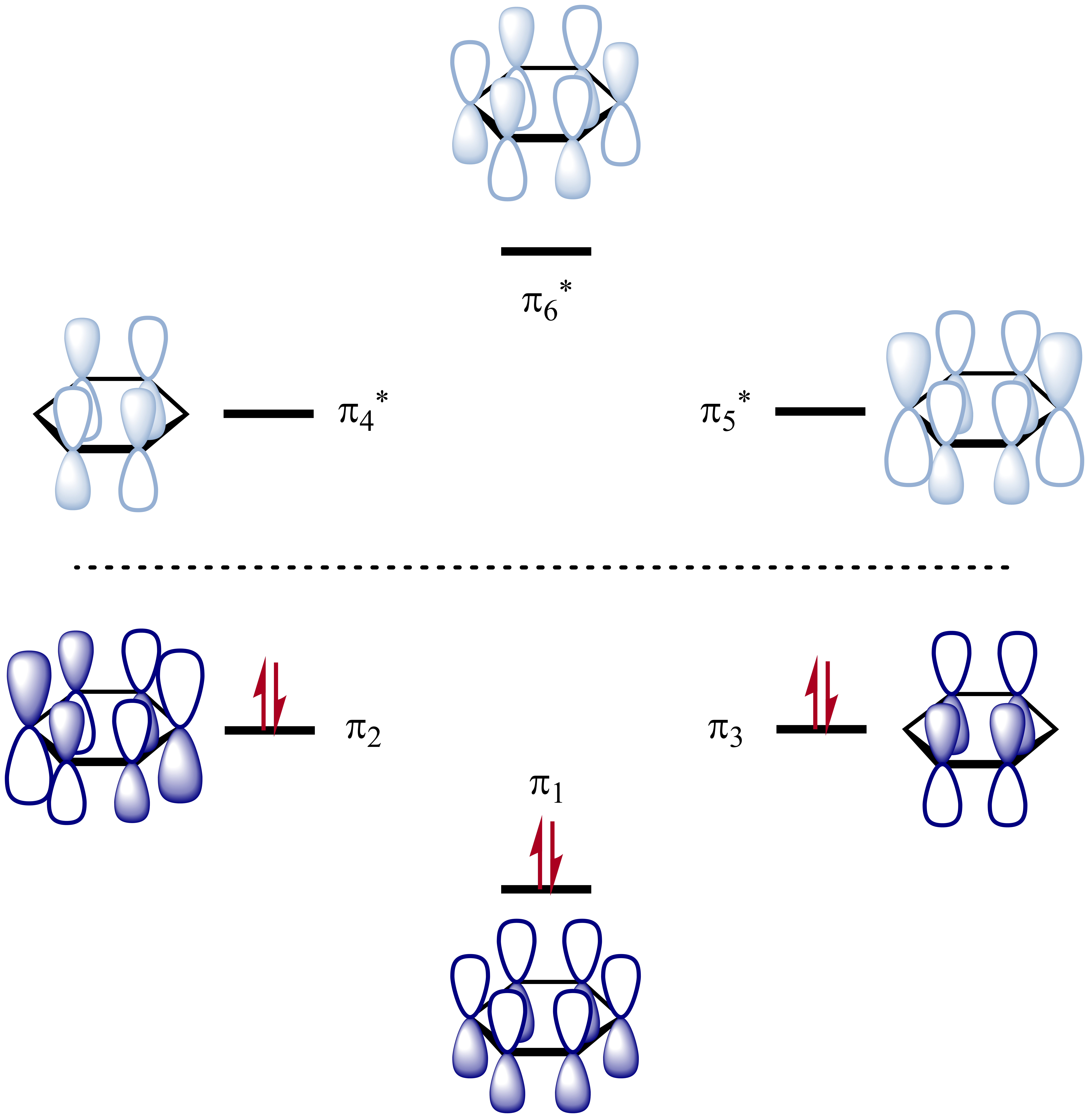
and Hund's rule. Cartoons showing overlapping p orbitals, like the one for benzene below, show the basis p atomic orbitals ''before'' they are combined to form molecular orbitals. In compliance with the Pauli exclusion principle, overlapping p orbitals ''do not'' result in the formation of one large MO containing more than two electrons.
Hückel MO theory is commonly used approach to obtain a zeroth order picture of delocalized π molecular orbitals, including the mathematical sign of the wavefunction at various parts of the molecule and the locations of nodal planes. It is particularly easy to apply for conjugated hydrocarbons and provides a reasonable approximation as long as the molecule is assumed to be planar with good overlap of p orbitals.
Stabilization energy
The quantitative estimation of stabilization from conjugation is notoriously contentious and depends on the implicit assumptions that are made when comparing reference systems or reactions. The energy of stabilization is known as the resonance energy when formally defined as the difference in energy between the real chemical species and the hypothetical species featuring localized π bonding that corresponds to the most stable resonance form. This energy cannot be measured, and a precise definition accepted by most chemists will probably remain elusive. Nevertheless, some broad statements can be made. In general, stabilization is more significant for cationic systems than neutral ones. For buta-1,3-diene, a crude measure of stabilization is theactivation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
for rotation of the C2-C3 bond. This places the resonance stabilization at around 6 kcal/mol. Comparison of heats of hydrogenation of 1,4-pentadiene and 1,3-pentadiene estimates a slightly more modest value of 3.5 kcal/mol. For comparison, allyl cation has a gas-phase rotation barrier of around 38 kcal/mol, a much greater penalty for loss of conjugation. Comparison of hydride ion affinities of propyl cation and allyl cation, corrected for inductive effects, results in a considerably lower estimate of the resonance energy at 20–22 kcal/mol. Nevertheless, it is clear that conjugation stabilizes allyl cation to a much greater extent than buta-1,3-diene. In contrast to the usually minor effect of neutral conjugation, aromatic stabilization can be considerable. Estimates for the resonance energy of benzene range from around 36–73 kcal/mol.
Generalizations and related concepts
 There are also other types of interactions that generalize the idea of interacting p orbitals in a conjugated system. The concept of ''hyperconjugation'' holds that certain σ bonds can also delocalize into a low-lying unoccupied orbital of a π system or an unoccupied p orbital. Hyperconjugation is commonly invoked to explain the stability of alkyl substituted radicals and carbocations. Hyperconjugation is less important for species in which all atoms satisfy the octet rule, but a recent computational study supports hyperconjugation as the origin of the increased stability of alkenes with a higher degree of substitution ( Zaitsev's rule).
''Homoconjugation'' is an overlap of two π-systems separated by a non-conjugating group, such as CH2. Unambiguous examples are comparatively rare in neutral systems, due to a comparatively minor energetic benefit that is easily overridden by a variety of other factors; however, they are common in cationic systems in which a large energetic benefit can be derived from delocalization of positive charge (''see the article on homoaromaticity for details.''). Neutral systems generally require constrained geometries favoring interaction to produce significant degrees of homoconjugation. In the example below, the carbonyl stretching frequencies of the IR spectra of the respective compounds demonstrate homoconjugation, or lack thereof, in the neutral ground state molecules.
Due to the partial π character of formally σ bonds in a cyclopropane ring, evidence for transmission of "conjugation" through cyclopropanes has also been obtained.
Two appropriately aligned π systems whose ends meet at right angles can engage in spiroconjugation or in homoconjugation across the spiro atom.
Vinylogy is the extension of a functional group through a conjugated organic bonding system, which transmits electronic effects.
There are also other types of interactions that generalize the idea of interacting p orbitals in a conjugated system. The concept of ''hyperconjugation'' holds that certain σ bonds can also delocalize into a low-lying unoccupied orbital of a π system or an unoccupied p orbital. Hyperconjugation is commonly invoked to explain the stability of alkyl substituted radicals and carbocations. Hyperconjugation is less important for species in which all atoms satisfy the octet rule, but a recent computational study supports hyperconjugation as the origin of the increased stability of alkenes with a higher degree of substitution ( Zaitsev's rule).
''Homoconjugation'' is an overlap of two π-systems separated by a non-conjugating group, such as CH2. Unambiguous examples are comparatively rare in neutral systems, due to a comparatively minor energetic benefit that is easily overridden by a variety of other factors; however, they are common in cationic systems in which a large energetic benefit can be derived from delocalization of positive charge (''see the article on homoaromaticity for details.''). Neutral systems generally require constrained geometries favoring interaction to produce significant degrees of homoconjugation. In the example below, the carbonyl stretching frequencies of the IR spectra of the respective compounds demonstrate homoconjugation, or lack thereof, in the neutral ground state molecules.
Due to the partial π character of formally σ bonds in a cyclopropane ring, evidence for transmission of "conjugation" through cyclopropanes has also been obtained.
Two appropriately aligned π systems whose ends meet at right angles can engage in spiroconjugation or in homoconjugation across the spiro atom.
Vinylogy is the extension of a functional group through a conjugated organic bonding system, which transmits electronic effects.
Conjugated cyclic compounds

Cyclic compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
s can be partly or completely conjugated. Annulenes, completely conjugated monocyclic hydrocarbons, may be aromatic, nonaromatic or antiaromatic.
Aromatic compounds
Compounds that have a monocyclic, planar conjugated system containing (4''n'' + 2) π-electrons for whole numbers ''n'' arearomatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
and exhibit an unusual stability. The classic example benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
has a system of six π electrons, which, together with the planar ring of C–C σ bonds containing 12 electrons and radial C–H σ bonds containing six electrons, forms the thermodynamically and kinetically stable '' benzene ring'', the common core of the benzenoid aromatic compounds. For benzene itself, there are two equivalent conjugated contributing Lewis structures (the so-called Kekulé structures) that predominate. The true electronic structure is therefore a quantum-mechanical combination (resonance hybrid) of these contributors, which results in the experimentally observed C–C bonds which are intermediate between single and double bonds and of equal strength and length. In the molecular orbital picture, the six p atomic orbitals of benzene combine to give six molecular orbitals. Three of these orbitals, which lie at lower energies than the isolated p orbital and are therefore net bonding in character (one molecular orbital is strongly bonding, while the other two are equal in energy but bonding to a lesser extent) are occupied by six electrons, while three destabilized orbitals of overall antibonding character remain unoccupied. The result is strong thermodynamic and kinetic aromatic stabilization. Both models describe rings of π electron density above and below the framework of C–C σ bonds.
Nonaromatic and antiaromatic compounds
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
, for example, possesses alternating single and double bonds. The molecule typically adopts a "tub" conformation. Because the p orbitals of the molecule do not align themselves well in this non-planar molecule, the π bonds are essentially isolated and not conjugated. The lack of conjugation allows the 8 π electron molecule to avoid antiaromaticity
Antiaromaticity is a chemical property of a cyclic molecule with a pi electron, π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. ...
, a destabilizing effect associated with cyclic, conjugated systems containing 4''n'' π (''n'' = 0, 1, 2, ...) electrons. This effect is due to the placement of two electrons into two degenerate nonbonding (or nearly nonbonding) orbitals of the molecule, which, in addition to drastically reducing the thermodynamic stabilization of delocalization, would either force the molecule to take on triplet diradical character, or cause it to undergo Jahn-Teller distortion to relieve the degeneracy. This has the effect of greatly increasing the kinetic reactivity of the molecule. Because of the lack of long-range interactions, cyclooctatetraene takes on a nonplanar conformation and is nonaromatic in character, behaving as a typical alkene. In contrast, derivatives of the cyclooctatetraene dication and dianion have been found to be planar experimentally, in accord with the prediction that they are stabilized aromatic systems with 6 and 10 π electrons, respectively. Because antiaromaticity is a property that molecules try to avoid whenever possible, only a few experimentally observed species are believed to be antiaromatic. Cyclobutadiene and cyclopentadienyl cation are commonly cited as examples of antiaromatic systems.
In pigments
In a conjugated pi-system, electrons are able to capture certain photons as the electrons resonate along a certain distance of p-orbitals - similar to how aradio antenna
In radio-frequency engineering, an antenna (American English) or aerial (British English) is an electronic device that converts an alternating electric current into radio waves (transmitting), or radio waves into an electric current (receivi ...
detects radio signals along its length. Typically, the more conjugated (longer) the pi-system is, the longer the wavelength of photon can be captured. Compounds whose molecules contain a sufficient number of conjugated bonds can absorb light in the visible region, and therefore appear colorful to the eye, usually appearing yellow or red.
Many dye
Juan de Guillebon, better known by his stage name DyE, is a French musician. He is known for the music video of the single "Fantasy
Fantasy is a genre of speculative fiction that involves supernatural or Magic (supernatural), magical ele ...
s make use of conjugated electron systems to absorb visible light
Light, visible light, or visible radiation is electromagnetic radiation that can be perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400–700 nanometres (nm ...
, giving rise to strong colors. For example, the long conjugated hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
chain in beta-carotene leads to its strong orange color. When an electron in the system absorbs a photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
of light of the right wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
, it can be promoted to a higher energy level. A simple model of the energy levels is provided by the quantum-mechanical problem of a one-dimensional particle in a box of length L, representing the movement of a π electron along a long conjugated chain of carbon atoms. In this model the lowest possible absorption energy corresponds to the energy difference between the highest occupied molecular orbital (HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
) and the lowest unoccupied molecular orbital (LUMO). For a chain of ''n'' C=C bonds or 2''n'' carbon atoms in the molecular ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
, there are 2''n'' π electrons occupying ''n'' molecular orbitals, so that the energy gap is
:
Since the box length ''L'' increases approximately linearly with the number of C=C bonds ''n'', this means that the energy Δ''E'' of a photon absorbed in the HOMO–LUMO transition is approximately proportional to 1/''n''. The photon wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
λ = ''hc''/Δ''E'' is then approximately proportional to ''n''. Although this model is very approximate, λ does in general increase with ''n'' (or ''L'') for similar molecules. For example, the HOMO–LUMO absorption wavelengths for conjugated butadiene, hexatriene and octatetraene are 217 nm, 252 nm and 304 nm respectively. However, for good numerical agreement of the particle in a box model with experiment, the single-bond/double-bond bond length alternations of the polyenes must be taken into account. Alternatively, one can use the Hückel method which is also designed to model the electronic structure of conjugated systems.
Many electronic transitions in conjugated π-systems are from a predominantly bonding molecular orbital (MO) to a predominantly antibonding MO (π to π*), but electrons from non-bonding lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s can also be promoted to a π-system MO (n to π*) as often happens in charge-transfer complexes. A HOMO to LUMO transition is made by an electron if it is allowed by the selection rules for electromagnetic transitions. Conjugated systems of fewer than eight conjugated double bonds absorb only in the ultraviolet region and are colorless to the human eye. With every double bond added, the system absorbs photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s of longer wavelength (and lower energy), and the compound ranges from yellow to red in color. Compounds that are blue or green typically do not rely on conjugated double bonds alone.
This absorption of light in the ultraviolet to visible spectrum can be quantified using ultraviolet–visible spectroscopy, and forms the basis for the entire field of photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 Nanometre, nm), visible ligh ...
.
Conjugated systems that are widely used for synthetic pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
s and dye
Juan de Guillebon, better known by his stage name DyE, is a French musician. He is known for the music video of the single "Fantasy
Fantasy is a genre of speculative fiction that involves supernatural or Magic (supernatural), magical ele ...
s are diazo and azo compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups).
IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted ...
s and phthalocyanine compounds.
Phthalocyanine compounds
Conjugated systems not only have low energy excitations in the visible spectral region but they also accept or donate electrons easily. Phthalocyanines, which, like Phthalocyanine Blue BN and Phthalocyanine Green G, often contain a transition metal ion, exchange an electron with the complexed transition metal ion that easily changes itsoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
. Pigments and dyes like these are charge-transfer complexes.
Porphyrins and similar compounds
Porphyrins have conjugated molecular ring systems ( macrocycles) that appear in manyenzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s of biological systems. As a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
, porphyrin forms numerous complexes with metallic ions like iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
in hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
that colors blood red. Hemoglobin transports oxygen to the cells of our bodies. Porphyrin–metal complexes often have strong colors. A similar molecular structural ring unit called chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenation, hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine. The name chlorin derives from chlorophyll. ...
is similarly complexed with magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
instead of iron when forming part of the most common forms of chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
molecules, giving them a green color. Another similar macrocycle unit is corrin, which complexes with cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
when forming part of cobalamin
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. One of eight B vitamins, it serves as a vital cofactor (biochemistry), cofactor in DNA synthesis and both fatty acid metabolism, fatty acid and amino a ...
molecules, constituting Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. One of eight B vitamins, it serves as a vital cofactor (biochemistry), cofactor in DNA synthesis and both fatty acid metabolism, fatty acid and amino a ...
, which is intensely red. The corrin unit has six conjugated double bonds but is not conjugated all the way around its macrocycle ring.
Chromophores
Conjugated systems form the basis ofchromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
s, which are light-absorbing parts of a molecule that can cause a compound to be colored. Such chromophores are often present in various organic compounds and sometimes present in polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s that are colored or glow in the dark. Chromophores often consist of a series of conjugated bonds and/or ring systems, commonly aromatic, which can include C–C, C=C, C=O, or N=N bonds.
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s including azo dyes
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C li ...
(also artificial food additives), compounds in fruits and vegetables (lycopene
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene (from the Neo-Latin '' Lycopersicon'', the name of a former tomato genus) is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and ve ...
and anthocyanidin
Anthocyanidins are common plant pigments, the aglycones of anthocyanins. They are based on the flavylium cation, an oxonium ion, with various groups substituent, substituted for its hydrogen atoms. They generally change color from red through p ...
s), photoreceptors of the eye, and some pharmaceutical compounds such as the following:
nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
s (PDots) are assembled from hydrophobic fluorescent conjugated polymers, along with amphiphilic polymers to provide water solubility. Pdots are important labels for single-molecule fluorescence microscopy, based on high brightness, lack of blinking
Blinking is a bodily function; it is a semi-autonomic rapid closing of the eyelid. A single blink is determined by the forceful closing of the eyelid or inactivation of the levator palpebrae superioris and the activation of the palpebral por ...
or dark fraction, and slow photobleaching
In optics, photobleaching (sometimes termed fading) is the photochemical alteration of a dye or a fluorophore molecule such that it is permanently unable to fluoresce. This is caused by cleaving of covalent bonds or non-specific reactions between ...
.
See also
* Conjugated microporous polymer * Cross-conjugation * Hyperconjugation * List of conjugated polymers *Metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be descr ...
* Polyene
*Resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
* Vinylogy
Notes
References
{{chemical bonds Physical organic chemistry * ″