|
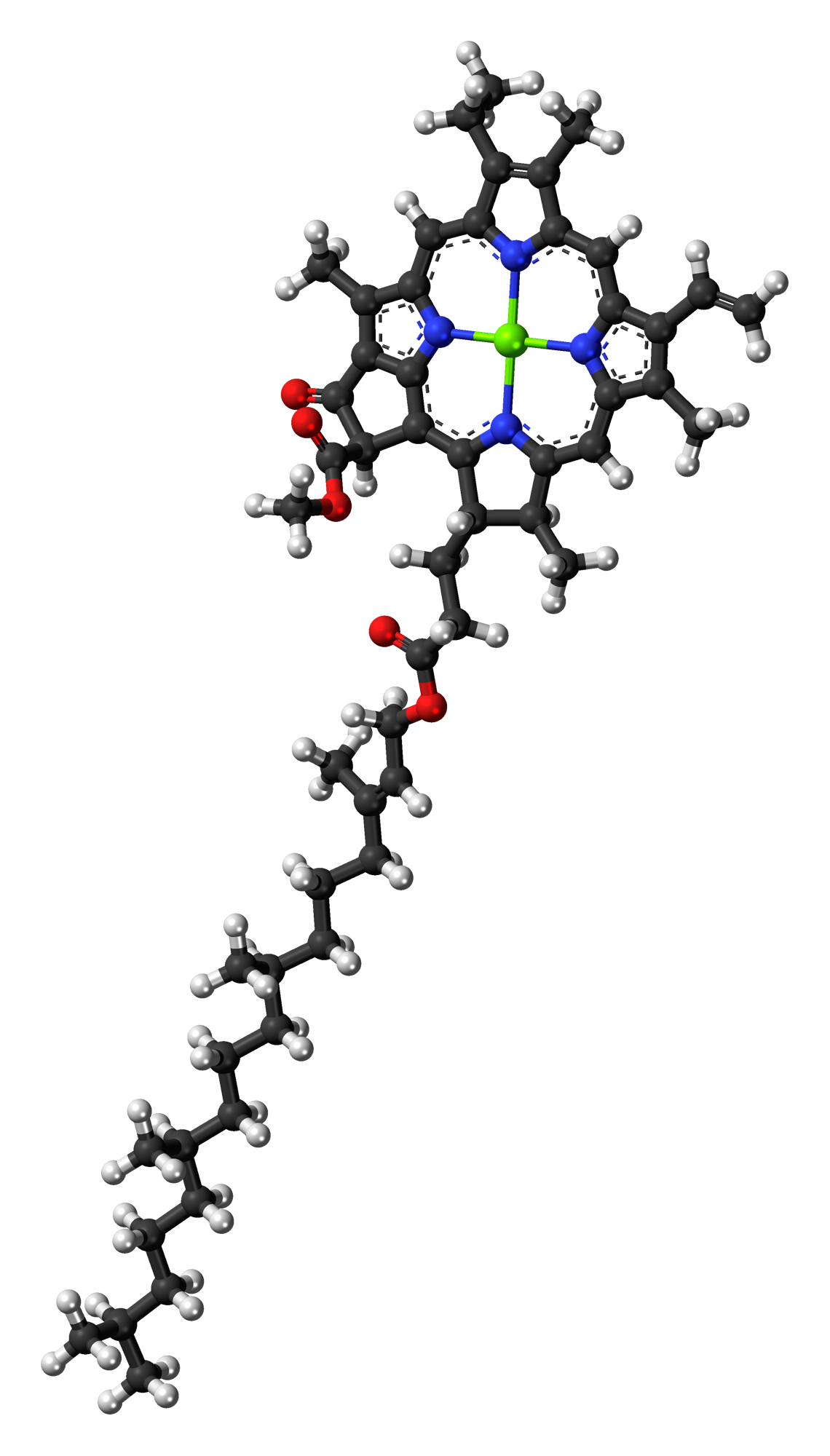
Chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenation, hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine. The name chlorin derives from chlorophyll. Chlorophylls are magnesium-containing chlorins and occur as photosynthetic pigments in chloroplasts. The term "chlorin" strictly speaking refers to only compounds with the same ring oxidation state as chlorophyll. Chlorins are excellent photosensitizing agents. Various synthetic chlorins analogues such as Temoporfin, m-tetrahydroxyphenylchlorin (mTHPC) and Talaporfin, mono-L-aspartyl chlorin e6 are effectively employed in experimental photodynamic therapy as photosensitizer. Chlorophylls The most abundant chlorin is the Photosynthesis, photosynthetic pigment chlorophyll. Chlorophylls have a fifth, ketone-containing ring unlike the chlorins. Diverse chlorophylls exists, such as Chlorophyll a, chlorophyll ''a'', Chlorophyll b, chlorophyll ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photodynamic Therapy
Photodynamic therapy (PDT) is a form of phototherapy involving light and a photosensitizing chemical substance used in conjunction with molecular oxygen to elicit cell death ( phototoxicity). PDT is used in treating acne, wet age-related macular degeneration, psoriasis, and herpes. It is used to treat malignant cancers, including head and neck, lung, bladder and skin. Advantages lessen the need for delicate surgery and lengthy recuperation and minimal formation of scar tissue and disfigurement. A side effect is the associated photosensitisation of skin tissue. Basics PDT applications involve three components: a photosensitizer, a light source and tissue oxygen. The wavelength of the light source needs to be appropriate for exciting the photosensitizer to produce radicals and/or reactive oxygen species. These are free radicals (Type I) generated through electron abstraction or transfer from a substrate molecule and highly reactive state of oxygen known as singlet oxygen ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriochlorophyll
Bacteriochlorophylls (BChl) are photosynthetic pigments that occur in various phototrophic bacteria. They were discovered by C. B. van Niel in 1932. They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanobacteria. Organisms that contain bacteriochlorophyll conduct photosynthesis to sustain their energy requirements, but the process is anoxygenic and does not produce oxygen as a byproduct. They use wavelengths of light not absorbed by plants or cyanobacteria. Replacement of with protons gives bacteriophaeophytin (BPh), the phaeophytin form. BacterioChlorophyll a.svg, bacteriochlorophyll ''a'' BacterioChlorophyll b.svg, bacteriochlorophyll ''b'' BacterioChlorophyll c.svg, bacteriochlorophyll ''c'' BacterioChlorophyll d.svg, bacteriochlorophyll ''d'' BacterioChlorophyll e.svg, bacteriochlorophyll ''e'' Bacteriochlorophyll f.svg, bacteriochlorophyll ''f'' BacterioChlorophyll g.svg, bacteriochlorophyll ''g'' Structure Bacteriochlorophyll ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sirohydrochlorin
Sirohydrochlorin is a tetrapyrrole macrocyclic metabolic intermediate in the biosynthesis of sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. It is also the biosynthetic precursor to cofactor F430, an enzyme which catalyzes the release of methane in the final step of methanogenesis. Structure Sirohydrochlorin was first isolated in the early 1970s when it was shown to be the metal-free form of the prosthetic group in the ferredoxin-nitrite reductase from spinach. Its chemical identity was established by spectroscopy and by total synthesis. Biosynthesis Sirohydrochlorin is derived from a tetrapyrrolic structural framework created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethylbilane to uroporphyrinogen III. The latter is the first macrocyclic intermediate common to haem, chlorophyll Chlorophyll is any of several related green pigments found in cyanobacteria and in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy from light. Those pigments are involved in oxygenic photosynthesis, as opposed to bacteriochlorophylls, related molecules found only in bacteria and involved in anoxygenic photosynthesis. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyrin
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis. The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons the porphyrin ring structure is a coordinated aromatic system. One result of the large conjugated system is that porphyrins absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives . Structure Porphyrin complexes consist of a square planar MN4 core. The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll A
} Chlorophyll ''a'' is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum. Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll ''a'' also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located. Distribution of chlorophyll ''a'' Chlorophyll ''a'' is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis. All oxygenic photosynthetic organisms use chloroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin Passivation (chemistry), passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium Salt (chemistry), salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight magnesium alloy, alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three Helium nucleus, helium nuclei to a carbon nucleus. When such stars explo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talaporfin
Talaporfin ( INN, also known as aspartyl chlorin, mono-L-aspartyl chlorin e6, NPe6, or LS11) is a chlorin based photosensitizer used in photodynamic therapy (PDT). It absorbs red light at 664-667 nm normally provided by a laser tuned to this wavelength. It was approved in Japan (in 2004) for PDT of lung cancer and marketed as Laserphyrin. References {{Chemotherapeutic agentsExtracorporeal Photo-Immunotherapy for Circulating Tumor Cells Photosensitizing agents Tetrapyrroles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocycle, macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III synthase, uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temoporfin
Temoporfin (INN) is a photosensitizer (based on chlorin) used in photodynamic therapy for the treatment of squamous cell carcinoma of the head and neck. It is marketed in the European Union under the brand name Foscan. The U.S. Food and Drug Administration The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ... (FDA) declined to approve Foscan in 2000. The EU approved its use in June 2001. Good results were obtained in 21 of 35 patients treated in Germany. It is photoactivated at 652 nm by red light. Patients can remain photosensitive for several weeks after treatment. References Further reading * {{Chemotherapeutic agents Photosensitizing agents Tetrapyrroles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapyrrole
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( or units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon atoms and one nitrogen atom. Tetrapyrroles are common cofactors in biochemistry and their biosynthesis and degradation feature prominently in the chemistry of life. Some tetrapyrroles form the active core of compounds with crucial biochemical roles in living systems, such as hemoglobin and chlorophyll. In these two molecules, in particular, the pyrrole macrocycle ring frames a metal atom, that forms a coordination compound with the pyrroles and plays a central role in the biochemical function of those molecules. Structure Linear tetrapyrroles (called bilanes) include: *Heme breakdown products (e.g., bilirubin, biliverdin) * Phycobilins (found in cyanobacteria) * Luciferins as found in dinoflagellates and euphausiid shrimps (krill) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |