chloralkali process on:
[Wikipedia]
[Google]
[Amazon]
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the

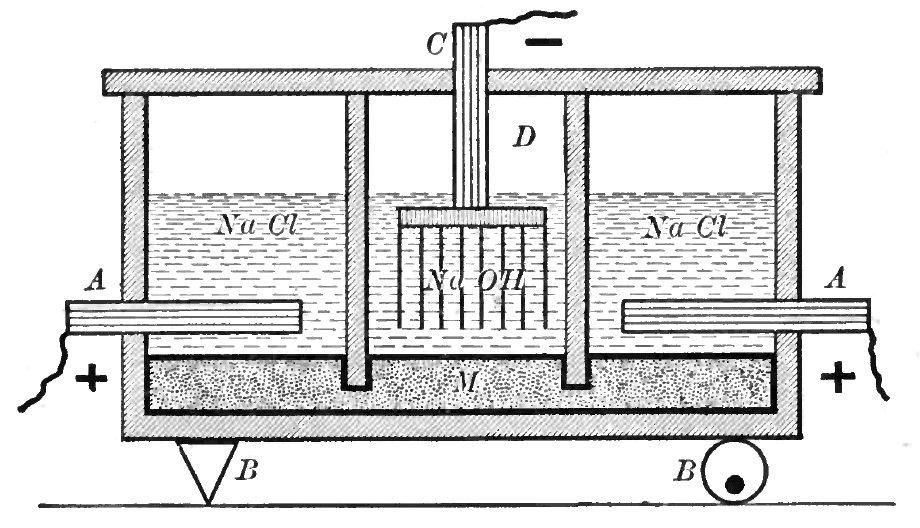 In the mercury-cell process, also known as the
In the mercury-cell process, also known as the
"Brine Electrolysis."
''Electrochemistry Encyclopedia.'' Cleveland: Case Western Reserve University.
Animation showing the membrane cell processAnimation showing the diaphragm cell process
{{electrolysis Chemical processes Electrolysis Industrial gases
electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
(NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
(caustic soda), which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. In 2022, this had increased to about 97 million tonnes. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.
Usually the process is conducted on a brine (an aqueous solution of concentrated NaCl), in which case sodium hydroxide (NaOH), hydrogen, and chlorine result. When using calcium chloride or potassium chloride, the products contain calcium or potassium instead of sodium. Related processes are known that use molten NaCl to give chlorine and sodium metal or condensed hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
to give hydrogen and chlorine.
The process has a high energy consumption, for example around of electricity per tonne of sodium hydroxide produced. Because the process yields equivalent amounts of chlorine and sodium hydroxide (two moles of sodium hydroxide per mole of chlorine), it is necessary to find a use for these products in the same proportion. For every mole of chlorine produced, one mole of hydrogen is produced. Much of this hydrogen is used to produce hydrochloric acid, ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
, hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
, or is burned for power and/or steam production.
History
The chloralkali process has been in use since the 19th century and is a primary industry in theUnited States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
, Western Europe
Western Europe is the western region of Europe. The region's extent varies depending on context.
The concept of "the West" appeared in Europe in juxtaposition to "the East" and originally applied to the Western half of the ancient Mediterranean ...
, and Japan
Japan is an island country in East Asia. Located in the Pacific Ocean off the northeast coast of the Asia, Asian mainland, it is bordered on the west by the Sea of Japan and extends from the Sea of Okhotsk in the north to the East China Sea ...
. It has become the principal source of chlorine during the 20th century. The diaphragm cell process and the mercury cell process have been used for over 100 years but are environmentally unfriendly through their use of asbestos and mercury, respectively. The membrane cell process, which was only developed in the past 60 years, is a superior method with its improved energy efficiency and lack of harmful chemicals.
Although the first formation of chlorine by the electrolysis of brine was attributed to chemist William Cruikshank in 1800, it was 90 years later that the electrolytic method was used successfully on a commercial scale. Industrial scale production began in 1892. In 1833, Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
formulated the laws that governed the electrolysis of aqueous solutions, and patents were issued to Cook and Watt in 1851 and to Stanley in 1853 for the electrolytic production of chlorine from brine.Process systems
Three production methods are in use. While the mercury cell method produces chlorine-free sodium hydroxide, the use of several tonnes of mercury leads to serious environmental problems. In a normal production cycle a few hundred pounds of mercury per year are emitted, which accumulate in the environment. Additionally, the chlorine and sodium hydroxide produced via the mercury-cell chloralkali process are themselves contaminated with trace amounts of mercury. The membrane and diaphragm method use no mercury, but the sodium hydroxide contains chlorine, which must be removed.Membrane cell
The most common chloralkali process involves the electrolysis ofaqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in wat ...
sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
(a brine) in a membrane cell. A membrane, such as Nafion, Flemion or Aciplex, is used to prevent the reaction between the chlorine and hydroxide ions.
Saturated brine is passed into the first chamber of the cell. Due to the higher concentration of chloride ions in the brine, the chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
ions are oxidised at the anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
, losing electrons to become chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
gas (A in figure):
:2Cl− → + 2 e−
At the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
, positive hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particl ...
s pulled from water molecules are reduced by the electrons provided by the electrolytic current, to hydrogen gas, releasing hydroxide ions into the solution (C in figure):
:2 + 2e− → H2 + 2OH−
The ion-permeable ion-exchange membrane at the center of the cell allows only the sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
ions (Na+) to pass to the second chamber where they react with the hydroxide ions to produce caustic soda (NaOH) (B in figure):
Na+ + OH− → NaOH
The overall reaction for the electrolysis of brine is thus:
:2NaCl + 2 → + + 2NaOH
Diaphragm cell
In the diaphragm cell process, there are two compartments separated by a permeable diaphragm, often made of asbestos fibers. Brine is introduced into the anode compartment and flows into the cathode compartment. Similarly to the membrane cell, chloride ions are oxidized at the anode to produce chlorine, and at the cathode, water is split into caustic soda and hydrogen. The diaphragm prevents the reaction of the caustic soda with the chlorine. A diluted caustic brine leaves the cell. The caustic soda must usually be concentrated to 50% and the salt removed. This is done using an evaporative process with about three tonnes of steam per tonne of caustic soda. The salt separated from the caustic brine can be used to saturate diluted brine. The chlorine contains oxygen and must often be purified by liquefaction and evaporation.Mercury cell
 In the mercury-cell process, also known as the
In the mercury-cell process, also known as the Castner–Kellner process
The Castner–Kellner process is a method of electrolysis on an aqueous alkali chloride solution (usually sodium chloride solution) to produce the corresponding alkali hydroxide, invented by American Hamilton Castner and Austrian Carl Kellner (mys ...
, the "outer" electrolytic cells each contain an anode immersed in brine, which floats on a layer of mercury. The "inner" cells each contain a cathode in a sodium hydroxide solution, floating on the same mercury layer. The walls dividing the cells have gaps below the surface of the mercury layer. This allows mercury to flow between cells, while preventing the aqueous solutions from doing so.
In the "outer" cell, chloride ions are oxidized at the anode, producing chlorine gas which bubbles out of the cell. The mercury layer acts as the cathode, here sodium ions in the brine are reduced and form an amalgam with the mercury. Once in the amalgam, sodium atoms are free to move to the "inner" cell.
In the "inner" cell, the mercury layer now acts as the anode. Sodium atoms in the amalgam are oxidized and enter aqueous solution. Meanwhile at the cathode, water is split into hydrogen gas and hydroxide ions.
Mercury cells are being phased out due to concerns about the high toxicity of mercury and mercury poisoning from mercury cell pollution such as occurred in Canada (see Ontario Minamata disease) and Japan (see Minamata disease).
Unpartitioned cell
The initial overall reaction produces hydroxide and also hydrogen and chlorine gases: :2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2 Without a membrane, the OH− ions produced at the cathode are free to diffuse throughout the electrolyte. As the electrolyte becomes morebasic
Basic or BASIC may refer to:
Science and technology
* BASIC, a computer programming language
* Basic (chemistry), having the properties of a base
* Basic access authentication, in HTTP
Entertainment
* Basic (film), ''Basic'' (film), a 2003 film
...
due to the production of OH−, less Cl2 emerges from the solution as it begins to disproportionate to form chloride and hypochlorite ions at the anode:
:Cl2 + 2 NaOH → NaCl + NaClO + H2O
The more opportunity the Cl2 has to interact with NaOH in the solution, the less Cl2 emerges at the surface of the solution and the faster the production of hypochlorite progresses. This depends on factors such as solution temperature, the amount of time the Cl2 molecule is in contact with the solution, and concentration of NaOH.
Likewise, as hypochlorite increases in concentration, chlorates are produced from them:
: 3 NaClO → NaClO3 + 2 NaCl
This reaction is accelerated at temperatures above about 60 °C. Other reactions occur, such as the self-ionization of water
The self-ionization of water (also autoionization of water, autoprotolysis of water, autodissociation of water, or simply dissociation of water) is an ionization reaction in properties of water, pure water or in an aqueous solution, in which a wa ...
and the decomposition of hypochlorite at the cathode, the rate of the latter depends on factors such as diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
and the surface area of the cathode in contact with the electrolyte.
If current is interrupted while the cathode is submerged, cathodes that are attacked by hypochlorites, such as those made from stainless steel, will dissolve in unpartitioned cells.
If producing hydrogen and oxygen gases is not a priority, the addition of 0.18% sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
or potassium chromate to the electrolyte will improve the efficiency of producing the other products.
Electrodes
Due to the corrosive nature of chlorine production, the anode (where the chlorine is formed) must be non-reactive and has been made from materials such asplatinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
metal, graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
(called plumbago in Faraday's time), or platinized titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
. A mixed metal oxide clad titanium anode (also called a dimensionally stable anode) is the industrial standard today. Historically, platinum, magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
, lead dioxide, manganese dioxide, and ferrosilicon
Ferrosilicon is an ferroalloy, alloy of iron and silicon. It has a typical silicon content of 15–90% by weight and a high proportion of iron silicides.
Production and reactions
Ferrosilicon is produced by reduction of silica or sand with coke ...
(13–15% silicon) have also been used as anodes. Platinum alloyed with iridium is more resistant to corrosion from chlorine than pure platinum. Unclad titanium cannot be used as an anode because it anodizes, forming a non-conductive oxide and passivates. Graphite will slowly disintegrate due to internal electrolytic gas production from the porous nature of the material and carbon dioxide forming due to carbon oxidation, causing fine particles of graphite to be suspended in the electrolyte that can be removed by filtration. The cathode (where hydroxide forms) can be made from unalloyed titanium, graphite, or a more easily oxidized metal such as stainless steel
Stainless steel, also known as inox, corrosion-resistant steel (CRES), or rustless steel, is an iron-based alloy that contains chromium, making it resistant to rust and corrosion. Stainless steel's resistance to corrosion comes from its chromi ...
or nickel.
Manufacturer associations
The interests of chloralkali product manufacturers are represented at regional, national and international levels by associations such as Euro Chlor and The World Chlorine Council.See also
* Electrochemical engineering * Gas diffusion electrode * Solvay process, a similar industrial method of making sodium carbonate fromcalcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
and sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
References
Further reading
* Bommaraju, Tilak V.; Orosz, Paul J.; Sokol, Elizabeth A.(2007)"Brine Electrolysis."
''Electrochemistry Encyclopedia.'' Cleveland: Case Western Reserve University.
External links
*Animation showing the membrane cell process
{{electrolysis Chemical processes Electrolysis Industrial gases