catalytic chain transfer on:
[Wikipedia]
[Google]
[Amazon]
Catalytic chain transfer (CCT) is a process that can be incorporated into radical polymerization to obtain greater control over the resulting products.
 In general, reactions of organic
In general, reactions of organic 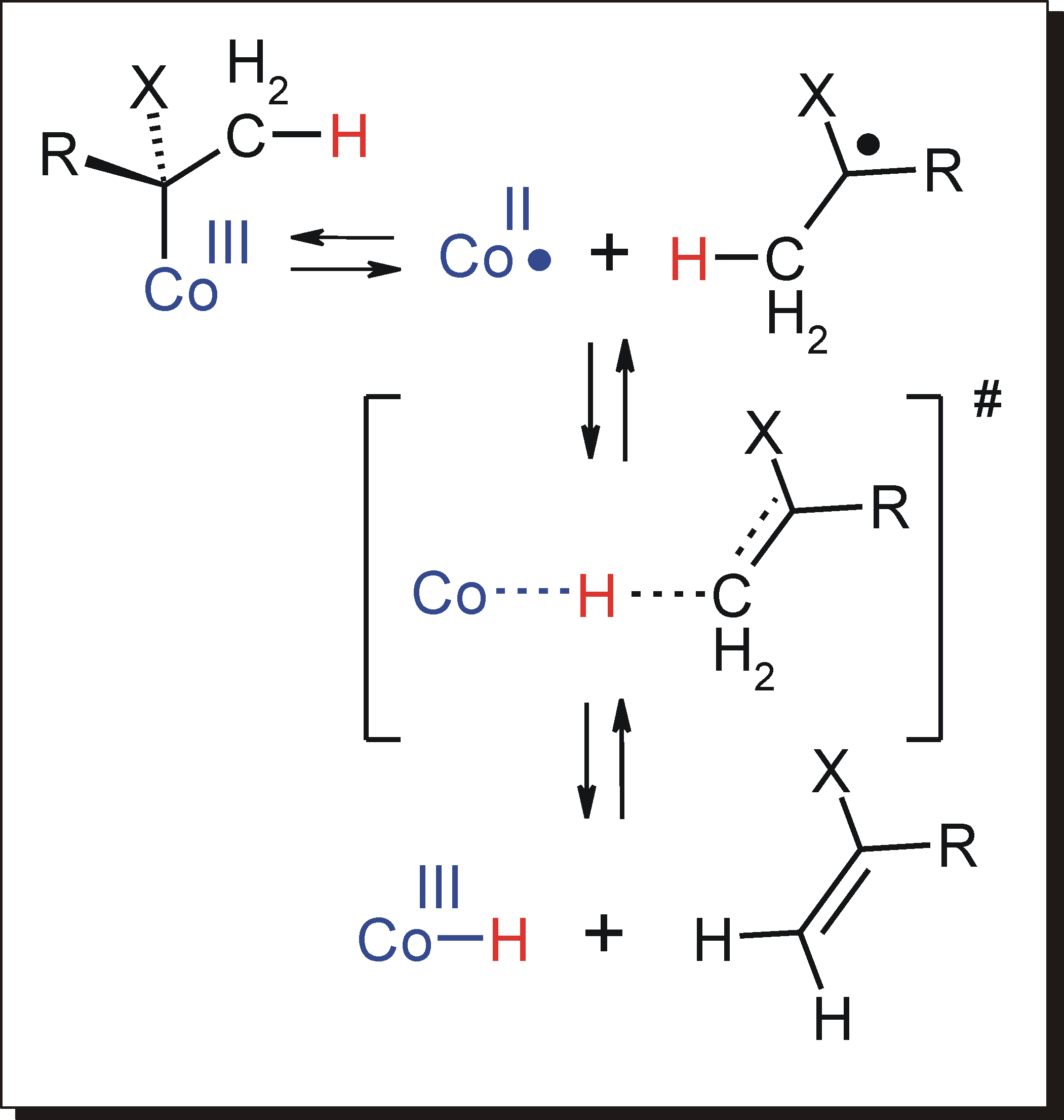 The olefin generating reaction 2 can become catalytic, and such catalytic chain transfer reactions are generally used to reduce the polymer molecular weight during the radical polymerization process. Mechanistically, catalytic chain transfer involves hydrogen atom transfer from the organic growing polymeryl radical to cobalt(II), thus leaving a polymer vinyl-end group and a cobalt-hydride species. The Co(por)(H) species has no cis-vacant site for direct insertion of a new olefinic monomer into the Co-H bond to finalize the chain-transfer process, and hence the required olefin insertion also proceeds via a radical pathway.
The best recognized chain transfer catalysts are low spin cobalt(II) complexes and organo-cobalt(III) species, which function as latent storage sites for organo-radicals required to obtain living radical polymerization by several pathways.
The major products of catalytic chain transfer polymerization are vinyl terminated polymer chains. One of the major drawbacks of the process is that catalytic chain transfer polymerization does not produce macromonomers of use in free radical polymerizations, but instead produces addition-fragmentation agents. When a growing polymer chain reacts with the addition fragmentation agent the radical end-group attacks the vinyl bond and forms a bond. However, the resulting product is so
The olefin generating reaction 2 can become catalytic, and such catalytic chain transfer reactions are generally used to reduce the polymer molecular weight during the radical polymerization process. Mechanistically, catalytic chain transfer involves hydrogen atom transfer from the organic growing polymeryl radical to cobalt(II), thus leaving a polymer vinyl-end group and a cobalt-hydride species. The Co(por)(H) species has no cis-vacant site for direct insertion of a new olefinic monomer into the Co-H bond to finalize the chain-transfer process, and hence the required olefin insertion also proceeds via a radical pathway.
The best recognized chain transfer catalysts are low spin cobalt(II) complexes and organo-cobalt(III) species, which function as latent storage sites for organo-radicals required to obtain living radical polymerization by several pathways.
The major products of catalytic chain transfer polymerization are vinyl terminated polymer chains. One of the major drawbacks of the process is that catalytic chain transfer polymerization does not produce macromonomers of use in free radical polymerizations, but instead produces addition-fragmentation agents. When a growing polymer chain reacts with the addition fragmentation agent the radical end-group attacks the vinyl bond and forms a bond. However, the resulting product is so
Introduction
Radical polymerization of vinyl monomers, like methyl (metha)acrylate of vinyl acetate is a common (industrial) method to prepare polymeric materials. One of the problems associated with this method is, however, that the radical polymerisationreaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
is so high that even at short reaction times the polymeric chains are exceedingly long. This has several practical disadvantages, especially for polymer processing (e.g. melt-processing). A solution to this problem is catalytic chain transfer, which is a way to make shorter polymer chains in radical polymerisation processes. The method involves adding a catalytic chain transfer agent to the reaction mixture of the monomer and the radical initiator.
Historical background
Boris Smirnov and Alexander Marchenko (USSR) discovered in 1975 that cobalt porphyrins are able to reduce the molecular weight of PMMA formed during radical polymerization of methacrylates. Later investigations showed that the cobalt dimethylglyoxime complexes were as effective as the porphyrin catalysts and also less oxygen sensitive.Janowicz, Andrew H. "Molecular weight control in free radical polymerizations" Issue date: Dec 12, 1989 Due to their lower oxygen sensitivity these catalysts have been investigated much more thoroughly than the porphyrin catalysts and are the catalysts actually used commercially.Process
 In general, reactions of organic
In general, reactions of organic free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
(•C(CH3)(X)R) with metal-centered radicals (M•) either produce an organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
complex (reaction 1) or a metal hydride (M-H) and an olefin (CH2=C(X)R) by the metallo radical M• abstracting a β-hydrogen from the organic radical •C(CH3)(X)R (reaction 2).
These organo-radical reactions with metal complexes provides several mechanisms to control radical polymerization of monomers CH2=CH(X). A wide range of metal-centered radicals and organo-metal complexes manifest at least a portion of these reactions. Various transition metal species, including complexes of Cr(I), Mo(III), Fe(I), V(0), Ti(III), and Co(II) have been demonstrated to control molecular weights in radical polymerization of olefins.
 The olefin generating reaction 2 can become catalytic, and such catalytic chain transfer reactions are generally used to reduce the polymer molecular weight during the radical polymerization process. Mechanistically, catalytic chain transfer involves hydrogen atom transfer from the organic growing polymeryl radical to cobalt(II), thus leaving a polymer vinyl-end group and a cobalt-hydride species. The Co(por)(H) species has no cis-vacant site for direct insertion of a new olefinic monomer into the Co-H bond to finalize the chain-transfer process, and hence the required olefin insertion also proceeds via a radical pathway.
The best recognized chain transfer catalysts are low spin cobalt(II) complexes and organo-cobalt(III) species, which function as latent storage sites for organo-radicals required to obtain living radical polymerization by several pathways.
The major products of catalytic chain transfer polymerization are vinyl terminated polymer chains. One of the major drawbacks of the process is that catalytic chain transfer polymerization does not produce macromonomers of use in free radical polymerizations, but instead produces addition-fragmentation agents. When a growing polymer chain reacts with the addition fragmentation agent the radical end-group attacks the vinyl bond and forms a bond. However, the resulting product is so
The olefin generating reaction 2 can become catalytic, and such catalytic chain transfer reactions are generally used to reduce the polymer molecular weight during the radical polymerization process. Mechanistically, catalytic chain transfer involves hydrogen atom transfer from the organic growing polymeryl radical to cobalt(II), thus leaving a polymer vinyl-end group and a cobalt-hydride species. The Co(por)(H) species has no cis-vacant site for direct insertion of a new olefinic monomer into the Co-H bond to finalize the chain-transfer process, and hence the required olefin insertion also proceeds via a radical pathway.
The best recognized chain transfer catalysts are low spin cobalt(II) complexes and organo-cobalt(III) species, which function as latent storage sites for organo-radicals required to obtain living radical polymerization by several pathways.
The major products of catalytic chain transfer polymerization are vinyl terminated polymer chains. One of the major drawbacks of the process is that catalytic chain transfer polymerization does not produce macromonomers of use in free radical polymerizations, but instead produces addition-fragmentation agents. When a growing polymer chain reacts with the addition fragmentation agent the radical end-group attacks the vinyl bond and forms a bond. However, the resulting product is so hindered
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
that the species undergoes fragmentation, leading eventually to telechelic species.
These addition fragmentation chain transfer agents do form graft copolymers with styrenic
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
and acrylate species however they do so by first forming block copolymers and then incorporating these block copolymers into the main polymer backbone. While high yields of macromonomers are possible with methacrylate monomers, low yields are obtained when using catalytic chain transfer agents during the polymerization of acrylate and styrenic monomers. This has been seen to be due to the interaction of the radical centre with the catalyst during these polymerization reactions.
Utility
The catalytic chain transfer process was commercialized very soon after its discovery. The initial commercial outlet was the production of chemically reactive macromonomers to be incorporated intopaint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s for the automotive industry. Federally mandated VOC restrictions are leading to the elimination of solvents from the automotive finishes and the lower molecular weight chain transfer products are often fluids. Incorporation of monomers such as glycidyl methacrylate or hydroxyethylmethacrylate
Hydroxyethylmethacrylate (also known as glycol methyacrylate) is the organic compound with the chemical formula . It is a colorless viscous liquid that readily polymerizes. Hydroxyethylmethacrylate is a monomer that is used to make various polymer ...
(HEMA) into the macromonomers aid curing processes. Macromonomers incorporating HEMA Hema may refer to:
* Hemā (mythology), a figure from Polynesian mythology
* HEMA (store), a Dutch chain of stores
* Hema (supermarket) (盒马), a supermarket chain in China
* Hema maps, an Australian map publisher
* Hema people, an ethnic group ...
can be effective in the dispersion of pigments in the paints. The chemistry is very effective under emulsion polymerisation conditions and has been used in the printing industry since 2000. The vinylic end group acts as an addition fragmentation agent and has been utilised to make multi block copolymers and derivatives used as stress relief agents in dental restoration by 3M.
See also
* Radical polymerization * Living polymerization *Cobalt Mediated Radical Polymerization Cobalt based catalysts, when used in radical polymerization, have several main advantages especially in slowing down the reaction rate, allowing for the synthesis of polymers with peculiar properties. As starting the reaction does need a real radi ...
References
{{Reflist, 2 Polymer chemistry Chemical processes