|
Radical Polymerization
In polymer chemistry, radical polymerization (RP) is a method of polymerization by which a polymer forms by the successive addition of a radical to building blocks ( repeat units). Radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating radical adds (nonradical) monomer units, thereby growing the polymer chain. Radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by radical polymerization. Radical polymerization is a type of chain polymerization, along with anionic, cationic and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polymer Chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.Hans-Heinrich Moretto, Manfred Schulz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Prepolymer
In polymer chemistry, the term prepolymer or pre-polymer, refers to a monomer or system of monomers that have been reacted to an intermediate-molecular mass state. This material is capable of further polymerization by reactive groups to a fully cured, high-molecular-mass state. As such, mixtures of reactive polymers with un-reacted monomers may also be referred to as pre-polymers. The term "pre-polymer" and "polymer precursor" may be interchanged. Polyurethane and polyurea prepolymers In polyurethane chemistry, prepolymers and oligomers are frequently produced and then further formulated into CASE applications - Coatings, Adhesives, Sealants, and Elastomers. An isocyanate (usually a diisocyanate) is reacted with a polyol. All types of polyol may in theory be used to produce polyurethane prepolymers. These then find use in CASE applications. When polyurethane dispersions are synthesized, a prepolymer is first produced usually modified with DMPA. In polyurea prepolymer productio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vinyl Group
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound containing that group, namely where R is any other group of atoms. An industrially important example is vinyl chloride, precursor to PVC, a plastic commonly known as ''vinyl''. Vinyl is one of the alkenyl functional groups. On a carbon skeleton, sp2-hybridized carbons or positions are often called vinylic. Allyls, acrylates and styrenics contain vinyl groups. (A styrenic crosslinker with two vinyl groups is called '' divinyl benzene''.) Vinyl polymers Vinyl groups can polymerize with the aid of a radical initiator or a catalyst, forming vinyl polymers. Vinyl polymers contain no vinyl groups. Instead they are saturated. The following table gives some examples of vinyl polymers. Synthesis and reactivity Vinyl derivatives are alke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative logarithm of one minus absorptance, as measured on a uniform sample". The term is used in many technical areas to quantify the results of an experimental measurement. While the term has its origin in quantifying the absorption of light, it is often entangled with quantification of light which is "lost" to a detector system through other mechanisms. What these uses of the term tend to have in common is that they refer to a logarithm of the ratio of a quantity of light incident on a sample or material to that which is detected after the light has interacted with the sample. The term absorption refers to the physical process of absorbing light, while absorbance does not always measure only absorption; it may measure attenuation (of transmitted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Initiation - Photolysis
Initiation is a rite of passage marking entrance or acceptance into a group or society. It could also be a formal admission to adulthood in a community or one of its formal components. In an extended sense, it can also signify a transformation in which the initiate is 'reborn' into a new role. Examples of initiation ceremonies might include Christian baptism or confirmation, Jewish bar or bat mitzvah, acceptance into a fraternal organization, secret society or religious order, or graduation from school or recruit training. A person taking the initiation ceremony in traditional rites, such as those depicted in these pictures, is called an ''initiate''. Characteristics William Ian Miller notes the role of ritual humiliation in comic ordering and testing. Mircea Eliade discussed initiation as a principal religious act by classical or traditional societies. He defined initiation as "a basic change in existential condition", which liberates man from profane time and history. "I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons with one target molecule that dissociates into two fragments. Here, “light” is broadly defined as radiation spanning the vacuum ultraviolet (VUV), ultraviolet (UV), visible, and infrared (IR) regions of the electromagnetic spectrum. To break covalent bonds, photon energies corresponding to visible, UV, or VUV light are typically required, whereas IR photons may be sufficiently energetic to detach ligands from coordination complexes or to fragment supramolecular complexes. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction, light phase, photochemical phase, or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Initiation - Thermal Decomp
Initiation is a rite of passage marking entrance or acceptance into a group or society. It could also be a formal admission to adulthood in a community or one of its formal components. In an extended sense, it can also signify a transformation in which the initiate is 'reborn' into a new role. Examples of initiation ceremonies might include Christian baptism or confirmation, Jewish bar or bat mitzvah, acceptance into a fraternal organization, secret society or religious order, or graduation from school or recruit training. A person taking the initiation ceremony in traditional rites, such as those depicted in these pictures, is called an ''initiate''. Characteristics William Ian Miller notes the role of ritual humiliation in comic ordering and testing. Mircea Eliade discussed initiation as a principal religious act by classical or traditional societies. He defined initiation as "a basic change in existential condition", which liberates man from profane time and history. "I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
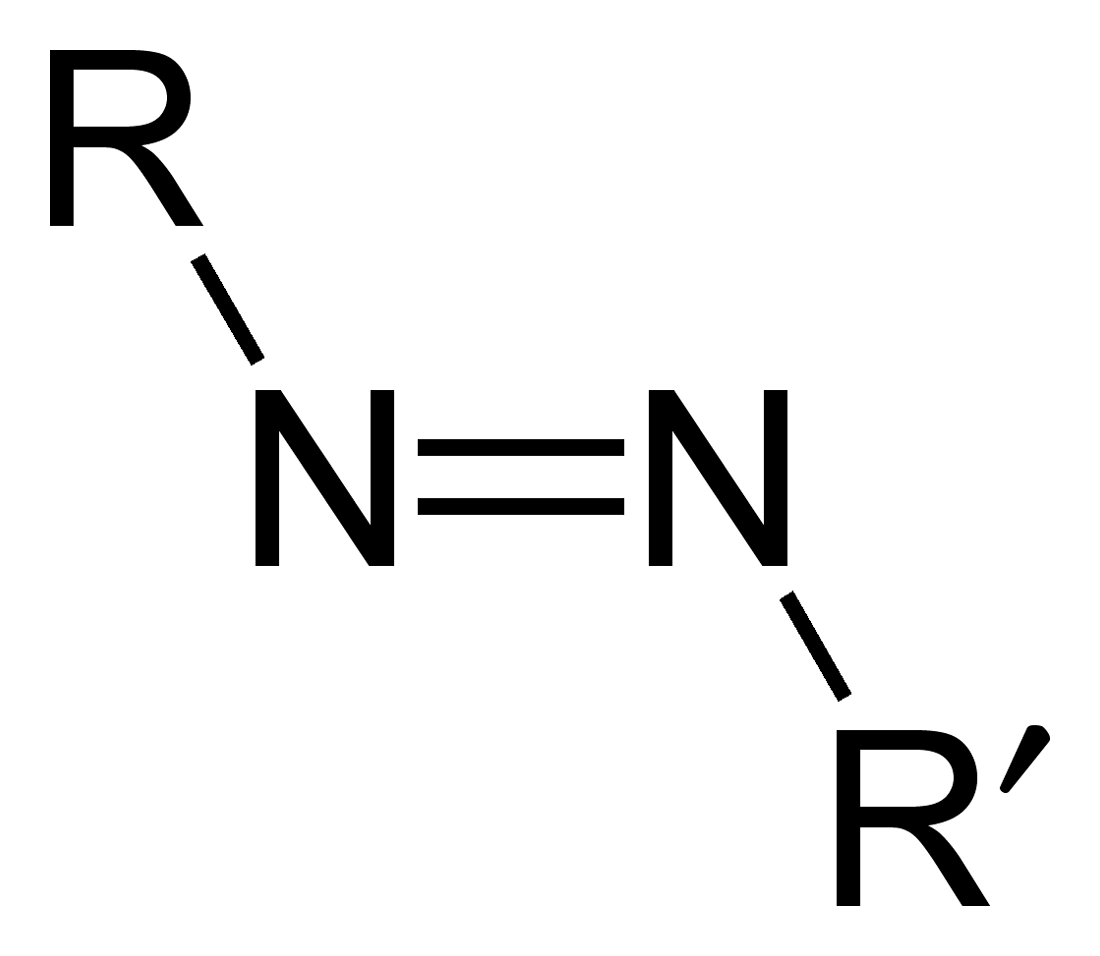
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined to each other and to adjacent elements through Single bond, single covalent bonds, denoted by dashes or lines. The group in a peroxide is often called the peroxide group, though some nomenclature discrepancies exist. This linkage is recognized as a common polyatomic ion, and exists in many molecules. General structure The characteristic structure of any regular peroxide is the oxygen–oxygen covalent single bond, which connects the two main atoms together. In the event that the molecule has no chemical Substituent, substituents, the peroxide group will have a [−2] Formal charge, net charge. Each oxygen atom has a charge of negative one, as 5 of its Valence electron, valence electrons remain in the outermost Atomic orbital, orbital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |