bicarbonate buffer system on:
[Wikipedia]
[Google]
[Amazon]
 The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of
section "Estimating plasma pH" in: : where: * p''K''''a'' H2CO3 is the negative logarithm (base 10) of the
National Academies of Sciences, Engineering, and Medicine. 1991. "Considerations in Contact Lens Use Under Adverse Conditions: Proceedings of a Symposium." Washington, DC: The National Academies Press. https://doi.org/10.17226/1773. The pH of tears shift throughout a waking day, rising "about 0.013 pH units/hour" until a prolonged closed-eye period causes the pH to fall again. Most healthy individuals have tear pH in the range of 7.0 to 7.7, where bicarbonate buffering is the most significant, but proteins and other buffering components are also present that are active outside of this pH range.
 The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
(H2CO3), bicarbonate ion (HCO), and carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(CO2) in order to maintain pH in the blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.
Blood is com ...
and duodenum
The duodenum is the first section of the small intestine in most vertebrates, including mammals, reptiles, and birds. In mammals, it may be the principal site for iron absorption.
The duodenum precedes the jejunum and ileum and is the shortest p ...
, among other tissues, to support proper metabolic
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the ...
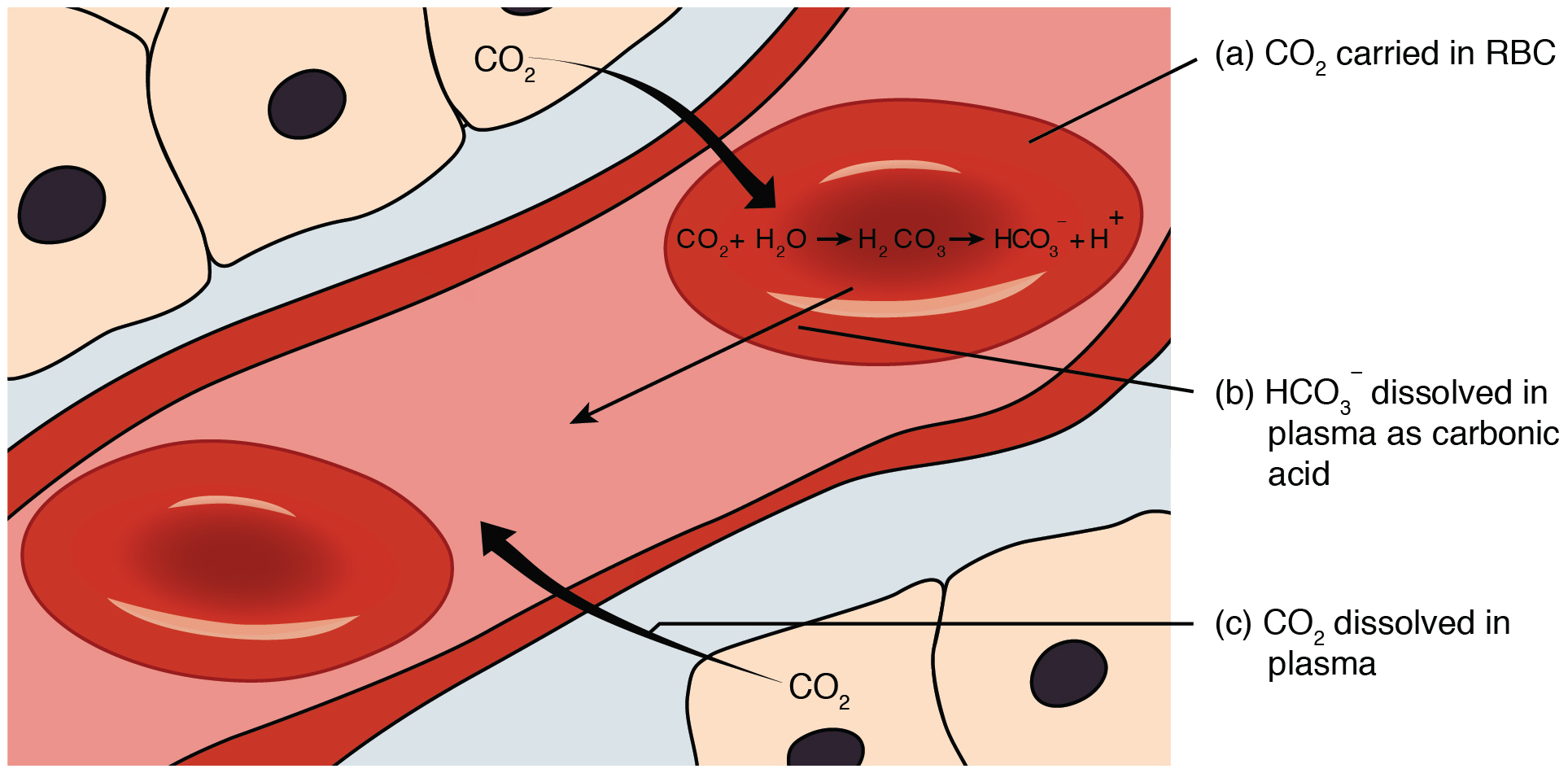
function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO ) and a hydrogen ion (H+) as shown in the following reaction:
As with any buffer system, the pH is balanced by the presence of both a weak acid (for example, H2CO3) and its conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reve ...
(for example, HCO) so that any excess acid or base introduced to the system is neutralized.
Failure of this system to function properly results in acid-base imbalance, such as acidemia
Acidosis is a biological process producing hydrogen ions and increasing their concentration in blood or body fluids. pH is the negative log of hydrogen ion concentration and so it is decreased by a process of acidosis.
Acidemia
The term acid ...
(pH < 7.35) and alkalemia (pH > 7.45) in the blood.
In systemic acid–base balance
In tissue,cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
produces carbon dioxide as a waste product; as one of the primary roles of the cardiovascular system
In vertebrates, the circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the body. It includes the cardiovascular system, or vascular system, that consists of the heart a ...
, most of this CO2 is rapidly removed from the tissues by its hydration to bicarbonate ion. The bicarbonate ion present in the blood plasma is transported to the lungs, where it is dehydrated back into CO2 and released during exhalation. These hydration and dehydration conversions of CO2 and H2CO3, which are normally very slow, are facilitated by carbonic anhydrase in both the blood and duodenum. While in the blood, bicarbonate ion serves to neutralize acid introduced to the blood through other metabolic processes (e.g. lactic acid
Lactic acid is an organic acid. It has the molecular formula C3H6O3. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as wel ...
, ketone bodies); likewise, any bases are neutralized by carbonic acid (H2CO3).
Regulation
As calculated by the Henderson–Hasselbalch equation, in order to maintain a normal pH of 7.4 in the blood (whereby the pKa of carbonic acid is 6.1 at physiological temperature), a 20:1 ratio of bicarbonate to carbonic acid must constantly be maintained; thishomeostasis
In biology, homeostasis (British English, British also homoeostasis; ) is the state of steady internal physics, physical and chemistry, chemical conditions maintained by organism, living systems. This is the condition of optimal functioning fo ...
is mainly mediated by pH sensors in the medulla oblongata
The medulla oblongata or simply medulla is a long stem-like structure which makes up the lower part of the brainstem. It is anterior and partially inferior to the cerebellum. It is a cone-shaped neuronal mass responsible for autonomic (involun ...
of the brain and probably in the kidney
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organ (anatomy), organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and rig ...
s, linked via negative feedback
Negative feedback (or balancing feedback) occurs when some function (Mathematics), function of the output of a system, process, or mechanism is feedback, fed back in a manner that tends to reduce the fluctuations in the output, whether caused ...
loops to effectors in the respiratory and renal systems. In the blood of most animals, the bicarbonate buffer system is coupled to the lung
The lungs are the primary Organ (biology), organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the Vertebral column, backbone on either side of the heart. Their ...
s via respiratory compensation, the process by which the rate and/or depth of breathing changes to compensate for changes in the blood concentration of CO2. By Le Chatelier's principle, the release of CO2 from the lungs pushes the reaction above to the left, causing carbonic anhydrase to form CO2 until all excess protons are removed. Bicarbonate concentration is also further regulated by renal compensation, the process by which the kidneys regulate the concentration of bicarbonate ions by secreting H+ ions into the urine while, at the same time, reabsorbing HCO ions into the blood plasma, or ''vice versa'', depending on whether the plasma pH is falling or rising, respectively.
Henderson–Hasselbalch equation
A modified version of the Henderson–Hasselbalch equation can be used to relate the pH ofblood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.
Blood is com ...
to constituents of the bicarbonate buffer system:page 556section "Estimating plasma pH" in: : where: * p''K''''a'' H2CO3 is the negative logarithm (base 10) of the
acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative property, quantitative measure of the acid strength, strength of an acid in Solution (chemistry), solution. I ...
of carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
. It is equal to 6.1.
* COis the concentration of bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
in the blood
* 2CO3is the concentration of carbonic acid in the blood
When describing arterial blood gas, the Henderson–Hasselbalch equation is usually quoted in terms of pCO2, the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, rather than H2CO3 concentration. However, these quantities are related by the equation:
:
where:
* 2CO3is the concentration of carbonic acid in the blood
* ''k''H CO2 is a constant including the solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
of carbon dioxide in blood. ''k''H CO2 is approximately 0.03 ( mmol/ L)/mmHg
A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high. Currently, it is defined as exactly , or approximately 1 torr = atmosphere = &nb ...
* ''p''CO2 is the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
in the blood
Combining these equations results in the following equation relating the pH of blood to the concentration of bicarbonate and the partial pressure of carbon dioxide:
:
where:
* pH is the acidity in the blood
* COis the concentration of bicarbonate in the blood, in mmol/ L
* ''p''CO2 is the partial pressure of carbon dioxide in the blood, in mmHg
Derivation of the Kassirer–Bleich approximation
The Henderson–Hasselbalch equation, which is derived from thelaw of mass action
In chemistry, the law of mass action is the proposition that the rate of a chemical reaction is directly proportional to the product of the activities or concentrations of the reactants. It explains and predicts behaviors of solutions in dy ...
, can be modified with respect to the bicarbonate buffer system to yield a simpler equation that provides a quick approximation of the H+ or HCO concentration without the need to calculate logarithms:
Since the partial pressure of carbon dioxide is much easier to obtain from measurement than carbonic acid, the Henry's law solubility constant – which relates the partial pressure of a gas to its solubility – for CO2 in plasma is used in lieu of the carbonic acid concentration. After solving for H+ and applying Henry's law, the equation becomes:
where ''K'' is the dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (''K''D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex ...
of carbonic acid, which is equal to 800 nmol/L (since ''K'' = 10−p''Ka''H2CO3 = 10−(6.1) ≈ 8.00×10−7 mol/L = 800 nmol/L).
After multiplying the constants (800 × 0.03 = 24) and solving for HCO, the equation is simplified to:
where:
* is the concentration of hydrogen ion in the blood, in nmol/ L
* COis the concentration of bicarbonate in the blood, in mmol/ L
* ''p''CO2 is the partial pressure of carbon dioxide in the blood, in mmHg
In other tissues
The bicarbonate buffer system plays a vital role in other tissues as well. In the human stomach and duodenum, the bicarbonate buffer system serves to both neutralizegastric acid
Gastric acid or stomach acid is the acidic component – hydrochloric acid – of gastric juice, produced by parietal cells in the gastric glands of the stomach lining. In humans, the pH is between one and three, much lower than most other a ...
and stabilize the intracellular pH of epithelial cells via the secretion of bicarbonate ion into the gastric mucosa
The gastric mucosa is the mucous membrane layer of the stomach, which contains the gastric pits, to which the gastric glands empty. In humans, it is about one mm thick, and its surface is smooth, soft, and velvety. It consists of simple secretor ...
. In patients with duodenal ulcers, ''Helicobacter pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, Flagellum#bacterial, flagellated, Bacterial cellular morphologies#Helical, helical bacterium. Mutants can have a rod or curved rod shape that exhibits l ...
'' eradication can restore mucosal bicarbonate secretion and reduce the risk of ulcer recurrence.
Tear buffering
Thetears
Tears are a clear liquid secreted by the lacrimal glands (tear gland) found in the eyes of all land mammals. Tears are made up of water, electrolytes, proteins, lipids, and mucins that form layers on the surface of eyes. The different types of ...
are unique among body fluid
Body fluids, bodily fluids, or biofluids, sometimes body liquids, are liquids within the Body (biology), body of an organism. In lean healthy adult men, the total body water is about 60% (60–67%) of the total Human body weight, body weight; it ...
s in that they are exposed to the environment. Much like other body fluids, tear fluid is kept in a tight pH range using the bicarbonate buffer system.Environmental Conditions and Tear ChemistryNational Academies of Sciences, Engineering, and Medicine. 1991. "Considerations in Contact Lens Use Under Adverse Conditions: Proceedings of a Symposium." Washington, DC: The National Academies Press. https://doi.org/10.17226/1773. The pH of tears shift throughout a waking day, rising "about 0.013 pH units/hour" until a prolonged closed-eye period causes the pH to fall again. Most healthy individuals have tear pH in the range of 7.0 to 7.7, where bicarbonate buffering is the most significant, but proteins and other buffering components are also present that are active outside of this pH range.
References
External links
* {{Renal physiology Electrolyte disturbances Buffer solutions