|
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid (). The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid (); and the conjugate acid of , t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
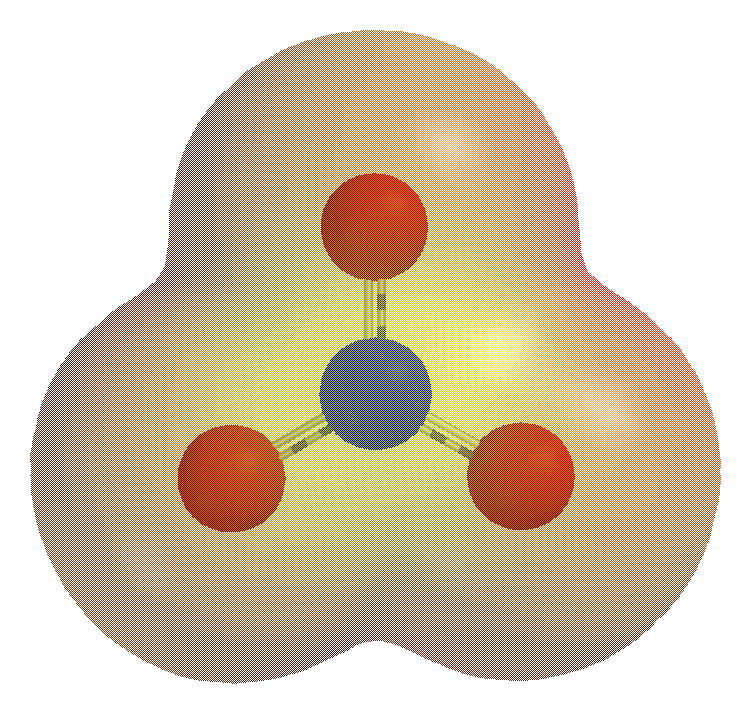
Carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, , the chief constituent of limestone (as well as the main component of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters. In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. Terminology in biochemical literature In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula . Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid. In physiology, carbon dioxide excreted by the lungs may be called ''volatile acid'' or ''respiratory acid''. Anh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffering Solution
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration increases by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphoterism
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used. Etymology and terminology Amphoteric is derived from the Greek word () meaning "both". Related words in acid-base chemistry are amphichromatic and amphichroic, both describing substances such as acid-base indicators which give one colour on reaction with an acid and another colour on reaction with a base. Amphiprotism Amphiprotism is exhibited by compounds with both Brønsted acidic and basic properties. A prime example is H2O. Amphiprotic molecules can either donate or accept a proton (). Amino acids (and proteins) are amphiprotic molecules because of their amine () and carboxylic acid () groups. Ampholytes Ampholytes are zwitterions ‒ molecules or ions that contain both acidic and basic functional groups. Amino acids have both a basic group and an acidic group . Of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Hyde Wollaston
William Hyde Wollaston (; 6 August 1766 – 22 December 1828) was an English chemist and physicist who is famous for discovering the chemical elements palladium and rhodium. He also developed a way to process platinum ore into malleable ingots,Melvyn C. UsselmanWilliam Hyde WollastonEncyclopædia Britannica, retrieved 31 March 2013 patented the camera lucida, and made contributions in electricity and spectroscopy. Life He was born in East Dereham in Norfolk, the son of the Francis Wollaston (1737–1815), a noted amateur astronomer, and his wife Althea Hyde. He was one of 17 children, but the family was financially well-off and he enjoyed an intellectually stimulating environment. He was educated privately (and remotely) at Charterhouse School from 1774 to 1778 then studied Sciences at Gonville and Caius College, Cambridge. In 1793 he obtained his doctorate (MD) in medicine from Cambridge University, and was a Fellow of his college from 1787 to 1828. He worked as a ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyatomic Ion
A polyatomic ion (also known as a molecular ion) is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that usually has a net charge that is not zero, or in special case of zwitterion wear spatially separated charges where the net charge may be variable depending on acidity conditions. The term molecule may or may not be used to refer to a polyatomic ion, depending on the definition used. The prefix ''poly-'' carries the meaning "many" in Greek, but even ions of two atoms are commonly described as polyatomic. In older literature, a polyatomic ion may instead be referred to as a '' radical'' (or less commonly, as a ''radical group''). In contemporary usage, the term ''radical'' refers to various free radicals, which are species that have an unpaired electron and need not be charged. A simple example of a polyatomic ion is the hydroxide ion, which consists of one oxygen atom and one hydrogen ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factitious Airs
Factitious airs was a term used for synthetic gases which emerged around 1670 when Robert Boyle coined the term upon isolating what is now understood to be hydrogen. ''Factitious'' means "artificial, not natural", so the term means "man-made gases". Background Robert Boyle coined the term Factitious Air upon isolating hydrogen in 1670. Henry Cavendish (1731–1810) used the term "factitious air" to refer to "any kind of air which is contained in other bodies in an unelastic state, and is produced from thence by art". An archaic definition from 1747 for the production of factitious air was defined as being caused by: "1- by flow Degrees from Putrefactions and Fermentations of all Kinds; or 2- more expeditiously by some Sorts of chymical Dissolutions of Bodies; or 3- and lastly, almost instantaneously by the Explosion of Gunpowder, and the Mixture or some Kinds of Bodies. Thus, if Paste or Dough with Leaven be placed in an exhausted Receiver, it will, after some Time, by Fermenta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reverse reaction. On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction. Because some acids can give multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: \text + \text \; \ce \; \text + \text Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which said that any compound that can give a proton to another compound is an acid, and the compound that receives the proton is a base. A proton is a subatomic particle in the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trivial Name
In chemistry, a trivial name is a non-systematic name for a chemical substance. That is, the name is not recognized according to the rules of any formal system of chemical nomenclature such as IUPAC inorganic or IUPAC organic nomenclature. A trivial name is not a formal name and is usually a common name. Generally, trivial names are not useful in describing the essential properties of the thing being named. Properties such as the molecular structure of a chemical compound are not indicated. And, in some cases, trivial names can be ambiguous or will carry different meanings in different industries or in different geographic regions (for example, a trivial name such as '' white metal'' can mean various things). Trivial names are simpler. As a result, a limited number of trivial chemical names are retained names, an accepted part of the nomenclature. Trivial names often arise in the common language; they may come from historic usages in, for example, alchemy. Many trivial name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |