Baylis–Hillman Reaction on:
[Wikipedia]
[Google]
[Amazon]
In  The reaction is named for Anthony B. Baylis and Melville E. D. Hillman, two of the chemists who developed the reaction at
The reaction is named for Anthony B. Baylis and Melville E. D. Hillman, two of the chemists who developed the reaction at
 If Hoffmann's model were correct, then the aldol addition would be the
If Hoffmann's model were correct, then the aldol addition would be the
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the Baylis–Hillman, Morita–Baylis–Hillman, or MBH reaction is a carbon–carbon bond-forming reaction between an activated alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
and a carbon electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
in the presence of a nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, such as a tertiary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
or phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
. The product is densely functionalized, joining the alkene at the α-position to a reduced
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox reacti ...
form of the electrophile (e.g. in the case of an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
, an allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
).
 The reaction is named for Anthony B. Baylis and Melville E. D. Hillman, two of the chemists who developed the reaction at
The reaction is named for Anthony B. Baylis and Melville E. D. Hillman, two of the chemists who developed the reaction at Celanese
Celanese Corporation, formerly known as Hoechst Celanese, is an American technology and specialty materials company headquartered in Irving, Texas. It is a Fortune 500 corporation. The company is the world's leading producer of acetic acid, pr ...
; and K. Morita, who published earlier work on the same.
The MBH reaction offers several advantages in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
:
# It combines easily prepared starting materials with high atom economy
Atom economy (atom efficiency/percentage) is the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced. The simplest definition was introduced by Barry Trost in 1991 and is equal to the rati ...
.
# It requires only mild conditions and does not require any transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s.
# Asymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
is possible from prochiral electrophiles.
# The product's dense functionalization enables many further transformations.
Its disadvantage is that the reaction is extremely slow.
Common reagents
The most frequently-used catalyst for the reaction is the tertiary amine DABCO (triethylenediamine); other known catalysts include 4-dimethylaminopyridine, DBU (diazabicycloundecene), and variousphosphines
Organophosphines are organophosphorus compounds with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphine ...
.
Reaction mechanism
, certain questions about MBH reaction's mechanism remain open. Hill and Isaacs performed the first kinetic experiments in the 1990s, discovering that thereaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
between acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group () linked to a nitrile (). It is an im ...
and acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
was first-order in each reactant and in the DABCO catalyst. α- Deuterated acrylonitrile exhibited no kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for t ...
, but the product appeared to catalyze its own generation.J. Phys. Org. Chem. 1990, 3, 285.
In a model Hoffmann proposed seven years prior, the reaction begins with 1,4-addition of the catalytic amine to the activated alkene. The resulting zwitterionic
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups.
:
(1,2-dipolar compounds, such as ylides, are sometimes excluded from t ...
aza-enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
ate undergoes aldol addition to the aldehyde. Intramolecular proton shift then generates the final MBH adduct, which eliminates the catalyst.
 If Hoffmann's model were correct, then the aldol addition would be the
If Hoffmann's model were correct, then the aldol addition would be the rate-limiting step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
, which accords with the absent kinetic isotope effect. However, Hoffman's mechanism rationalizes neither the product's autocatalysis nor (in the reaction of aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
aldehydes with acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain ...
s) the considerable generation of a dioxanone Dioxanone may refer to:
*Trimethylene carbonate (1,3-dioxan-2-one)
*p-Dioxanone, ''p''-Dioxanone (1,4-dioxan-2-one)
{{Short pages monitor
 The MBH reaction is extremely general. In most cases the electrophile is an aldehyde,
The MBH reaction is extremely general. In most cases the electrophile is an aldehyde,  In aprotic solvents, the reaction rate is even slower, although recovery is possible with protic additives (e.g. alcohols and carboxylic acids).
At such low rates, the activity of the substrates may induce competing side-reactions:
In aprotic solvents, the reaction rate is even slower, although recovery is possible with protic additives (e.g. alcohols and carboxylic acids).
At such low rates, the activity of the substrates may induce competing side-reactions:  Due to the highly negative volume of activation, sluggish Baylis–Hillman reactions — including ketonic ones — can be realized by conducting the reaction under high pressure (up to 20 kbar).
Due to the highly negative volume of activation, sluggish Baylis–Hillman reactions — including ketonic ones — can be realized by conducting the reaction under high pressure (up to 20 kbar).
 In the sila-MBH reaction, α-silylated vinyl aryl ketones couple to aldehydes in the presence of catalytic TTMPP, a large triaryl
In the sila-MBH reaction, α-silylated vinyl aryl ketones couple to aldehydes in the presence of catalytic TTMPP, a large triaryl
 The Rauhut–Currier reaction is a
The Rauhut–Currier reaction is a
 Likewise, activated acetylenes can undergo
Likewise, activated acetylenes can undergo 
 A related hydrazide auxiliary is the chiral acryloylhydrazide, which reacts diastereoselectively with aldehydes. Both diastereomers could be obtained with different choice of solvents (DMSO vs. mixed THF and H2O), suggesting that the transition structure conformation is solvent-influenced.
Chiral allenes and imines can also be employed for an asymmetric DABCO-catalyzed ''aza''-MBH reaction. Optically active 10-phenylsulfonylisobornyl buta-2,3-dienoate reacts with an aryl imine to afford α-allenylamine in a diastereoselective manner (37–57% yield).
A related hydrazide auxiliary is the chiral acryloylhydrazide, which reacts diastereoselectively with aldehydes. Both diastereomers could be obtained with different choice of solvents (DMSO vs. mixed THF and H2O), suggesting that the transition structure conformation is solvent-influenced.
Chiral allenes and imines can also be employed for an asymmetric DABCO-catalyzed ''aza''-MBH reaction. Optically active 10-phenylsulfonylisobornyl buta-2,3-dienoate reacts with an aryl imine to afford α-allenylamine in a diastereoselective manner (37–57% yield).

 The phenolic oxygen of β-ICD was shown to be important in the reaction, implying that β-ICD acts as a Brønsted–Lowry acid, not just a nucleophile.
Cyclopentenone and various aromatic and aliphatic aldehydes undergo an asymmetric reaction using Fu's planar chiral DMAP catalyst in isopropanol (54–96% yield, 53–98% ee). In this case, magnesium iodide as a Lewis acid cocatalyst was required to accelerate the reaction.
The phenolic oxygen of β-ICD was shown to be important in the reaction, implying that β-ICD acts as a Brønsted–Lowry acid, not just a nucleophile.
Cyclopentenone and various aromatic and aliphatic aldehydes undergo an asymmetric reaction using Fu's planar chiral DMAP catalyst in isopropanol (54–96% yield, 53–98% ee). In this case, magnesium iodide as a Lewis acid cocatalyst was required to accelerate the reaction.
 ''P''-Chiral phosphines have been investigated.
Simple diamines can also be employed as MBH catalysts. Methyl vinyl ketone and various substituted benzaldehydes were found to undergo asymmetric MBH reaction. The chiral pyrrolidine catalyst was effective for ortho- and para-substituted electron-deficient benzaldehydes (75–99% yield, 8–73% ee).
''P''-Chiral phosphines have been investigated.
Simple diamines can also be employed as MBH catalysts. Methyl vinyl ketone and various substituted benzaldehydes were found to undergo asymmetric MBH reaction. The chiral pyrrolidine catalyst was effective for ortho- and para-substituted electron-deficient benzaldehydes (75–99% yield, 8–73% ee).
 Chiral phosphine MBH catalysts often contain Brønsted–Lowry acid moieties in their backbones. For example, chiral phosphines containing a Lewis base, a Brønsted–Lowry acid, and an acid-activated Brønsted–Lowry base were developed for an asymmetric ''aza''-MBH reaction (86–96% yield, 79–92% ee). The Brønsted–Lowry acid and base moieties were proposed to be involved in the stabilization of zwitterionic species in a stereoselective manner.
Chiral phosphine MBH catalysts often contain Brønsted–Lowry acid moieties in their backbones. For example, chiral phosphines containing a Lewis base, a Brønsted–Lowry acid, and an acid-activated Brønsted–Lowry base were developed for an asymmetric ''aza''-MBH reaction (86–96% yield, 79–92% ee). The Brønsted–Lowry acid and base moieties were proposed to be involved in the stabilization of zwitterionic species in a stereoselective manner.
 BINOL-derived chiral phosphine catalyst is also effective for an asymmetric aza-MBH reaction of N-tosyl imines with activated alkenes such as methyl vinyl ketone and phenyl acrylate.
In addition, a distinct class of chiral phosphine-
BINOL-derived chiral phosphine catalyst is also effective for an asymmetric aza-MBH reaction of N-tosyl imines with activated alkenes such as methyl vinyl ketone and phenyl acrylate.
In addition, a distinct class of chiral phosphine-
 Complex of metal salt and chiral ligand is a viable strategy, too. La(OTf)3 and camphor-derived chiral ligands could induce enantioselectivity in a DABCO-catalyzed MBH reaction of various aldehydes and acrylates (25–97% yield, 6–95% ee). For these cases, multidentate ligands were usually employed to chelate with the metal, which activates both the zwitterionic enolate and the aldehyde.
Complex of metal salt and chiral ligand is a viable strategy, too. La(OTf)3 and camphor-derived chiral ligands could induce enantioselectivity in a DABCO-catalyzed MBH reaction of various aldehydes and acrylates (25–97% yield, 6–95% ee). For these cases, multidentate ligands were usually employed to chelate with the metal, which activates both the zwitterionic enolate and the aldehyde.
 La(O-iPr)3 and BINOL-derived ligand system, in conjunction with catalytic DABCO, also works for an asymmetric aza-MBH reaction of various N-diphenylphosphinoyl imines and methyl acrylate. Aryl, heteroaryl, and alkenyl imines were all suitable for good yield and enantioselectivity.
Chiral palladium(II) pincer complexes function as Lewis acid in the enantioselective DABCO-catalyzed aza-MBH reaction of acrylonitrile and various tosyl imines to afford functionalized α-methylene-β-aminonitriles (75–98% yield, 76–98% ee). Silver acetate is required to activate the palladium bromide precatalyst in the catalytic cycle.
La(O-iPr)3 and BINOL-derived ligand system, in conjunction with catalytic DABCO, also works for an asymmetric aza-MBH reaction of various N-diphenylphosphinoyl imines and methyl acrylate. Aryl, heteroaryl, and alkenyl imines were all suitable for good yield and enantioselectivity.
Chiral palladium(II) pincer complexes function as Lewis acid in the enantioselective DABCO-catalyzed aza-MBH reaction of acrylonitrile and various tosyl imines to afford functionalized α-methylene-β-aminonitriles (75–98% yield, 76–98% ee). Silver acetate is required to activate the palladium bromide precatalyst in the catalytic cycle.

 While simple thiourea requires a nucleophilic catalyst in conjunction, bifunctional catalysts such as phosphine-thioureas can be used alone for asymmetric MBH reactions. For example, various acrylates and aromatic aldehydes react in the presence of these catalysts to afford either enantiomeric MBH adducts (32–96% yield, 9–77% ee).
While simple thiourea requires a nucleophilic catalyst in conjunction, bifunctional catalysts such as phosphine-thioureas can be used alone for asymmetric MBH reactions. For example, various acrylates and aromatic aldehydes react in the presence of these catalysts to afford either enantiomeric MBH adducts (32–96% yield, 9–77% ee).
 MBH reaction can involve proline derivative as a cocatalyst. It was proposed that imidazole nucleophilic catalyst and proline effect the reaction via iminium intermediate. With (S)-proline and DABCO, α-amido sulfones and α,β-unsaturated aldehydes undergo a highly enantioselective aza-MBH reaction (46–87% yield, E/Z 10:1–19:1, 82–99% ee).
MBH reaction can involve proline derivative as a cocatalyst. It was proposed that imidazole nucleophilic catalyst and proline effect the reaction via iminium intermediate. With (S)-proline and DABCO, α-amido sulfones and α,β-unsaturated aldehydes undergo a highly enantioselective aza-MBH reaction (46–87% yield, E/Z 10:1–19:1, 82–99% ee).




Scope and limitations
 The MBH reaction is extremely general. In most cases the electrophile is an aldehyde,
The MBH reaction is extremely general. In most cases the electrophile is an aldehyde, ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
(but see below), or imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
(latterly the ''aza''-Baylis–Hillman reaction); but allyl halides, alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
s, and epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
s are also possible. Using an allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
instead of a simple alkene as the precursor gives an intermediate that can react at the γ carbon rather than at the α.
At the same time, it can be challenging to develop suitable reaction conditions. The reaction is slow (times of a fortnight
A fortnight is a unit of time equal to 14 days (two weeks). The word derives from the Old English term , meaning "" (or "fourteen days", since the Anglo-Saxons counted by nights).
Astronomy and tides
In astronomy, a ''lunar fortnight'' is hal ...
or longer are not uncommon, even with 25-100 mol % catalyst), especially with (as alkene) β-substituted activated olefins, vinyl sulfones, or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s; or (as electrophile) hindered aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
aldehydes or electron-rich
Electron-rich is jargon that is used in multiple related meanings with either or both kinetic and thermodynamic implications:
* with regards to electron-transfer, electron-rich species have low ionization energy and/or are reducing agents. Tetr ...
benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
s. Ketones are generally not reactive enough under ordinary conditions to take part in a synthetically useful manner. For example, reaction between sterically hindered ''t''-butyl acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain ...
and benzaldehyde with catalytic DABCO
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in ...
in the absence of solvent required 4 weeks to give ''moderate'' conversion to the final product.
 In aprotic solvents, the reaction rate is even slower, although recovery is possible with protic additives (e.g. alcohols and carboxylic acids).
At such low rates, the activity of the substrates may induce competing side-reactions:
In aprotic solvents, the reaction rate is even slower, although recovery is possible with protic additives (e.g. alcohols and carboxylic acids).
At such low rates, the activity of the substrates may induce competing side-reactions: acrolein
Acrolein (systematic name: propenal) is the simplest unsaturated aldehyde. It is a colorless liquid with a foul and acrid aroma. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fat ...
s also oligomerize and allenoates cycloadd. Allyl-halide and alkyl-epoxide electrophiles also often prove unruly. The MBH reaction of an aryl vinyl ketone with an aldehyde is not straightforward (but see ), since the reactive aryl vinyl ketone readily undergoes Michael addition
In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a c ...
to another molecule of the aryl vinyl ketone, which then adds to the aldehyde to form a double-MBH adduct.
 Due to the highly negative volume of activation, sluggish Baylis–Hillman reactions — including ketonic ones — can be realized by conducting the reaction under high pressure (up to 20 kbar).
Due to the highly negative volume of activation, sluggish Baylis–Hillman reactions — including ketonic ones — can be realized by conducting the reaction under high pressure (up to 20 kbar).
Variants
Sila-MBH reaction
 In the sila-MBH reaction, α-silylated vinyl aryl ketones couple to aldehydes in the presence of catalytic TTMPP, a large triaryl
In the sila-MBH reaction, α-silylated vinyl aryl ketones couple to aldehydes in the presence of catalytic TTMPP, a large triarylphosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
reagent. The zwitterionic enolate produced upon addition of nucleophilic catalyst to the enone adds to an aldehyde carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
to generate an alkoxide. This alkoxide undergoes a subsequent 1,3-Brook rearrangement
In organic chemistry the Brook rearrangement refers to any ,''n''carbon to oxygen silyl migration. The Rearrangement reaction, rearrangement was first observed in the late 1950s by Canadian chemist Adrian Gibbs Brook (1924–2013), after which ...
and elimination cascade to afford a siloxy-methylene enone and release the catalyst.
Rauhut–Currier reaction
 The Rauhut–Currier reaction is a
The Rauhut–Currier reaction is a vinylogous
In organic chemistry, vinylogy is the transmission of electronic effects through a conjugated organic bonding system. The concept was introduced in 1926 by Ludwig Claisen to explain the acidic properties of formylacetone and related ketoald ...
analogoue of the MBH reaction, in which the electrophile is a Michael acceptor
In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a c ...
, not an aldehyde or an imine. Intermolecular Rauhut–Currier reactions typically exhibit poor chemoselectivity Chemoselectivity is the preferential reaction of a chemical reagent with one of two or more different functional groups.
In a chemoselective system, a reagent in the presence of an aldehyde and an ester would mostly target the aldehyde, even if it ...
, because the reaction couples two activated alkenes, but intramolecular Rauhut–Currier reactions have been employed. For example, cyclization of α,β-unsaturated aldehydes can be performed in the presence of proline derivative and acetic acid, affording enantioenriched products.
Tandem strategies
As mentioned above, the slow rate of the MBH reaction often enables side-reactions on its activated substrates. In tandem reaction strategies, this is a virtue, for it enables syntheses with high atom economy. For example, in the three-component coupling of aldehydes, amines, and activated alkenes, the aldehyde reacts with the amine to produce animine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
prior to forming the ''aza''-MBH adduct, as in the reaction of aryl aldehydes, diphenylphosphinamide, and methyl vinyl ketone
Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid wi ...
, in the presence of TiCl4, triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ...
, and triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
:
 Likewise, activated acetylenes can undergo
Likewise, activated acetylenes can undergo conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polar ...
and remain an activated alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
for the MBH reaction, as in the following enantioselective cyclization reaction
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
in which a phenolate
Phenolates (also called phenoxides) are anions, salt (chemistry), salts, and esters of phenols, containing the phenolate ion. They may be formed by reaction of phenols with strong base.
Properties
Alkali metal phenolates, such as sodium phenoxi ...
nucleophile adds to a functionalized enyne
An enyne is an organic compound containing a double bond (alkene) and a triple bond (alkyne). It is called a conjugated enyne when the double and triple bonds are conjugated.
The term is a contraction of the terms alkene and alkyne.
The si ...
before ''aza''-MBH ring closure catalyzed by a chiral amine base.

Asymmetric synthesis
Chiral auxiliaries
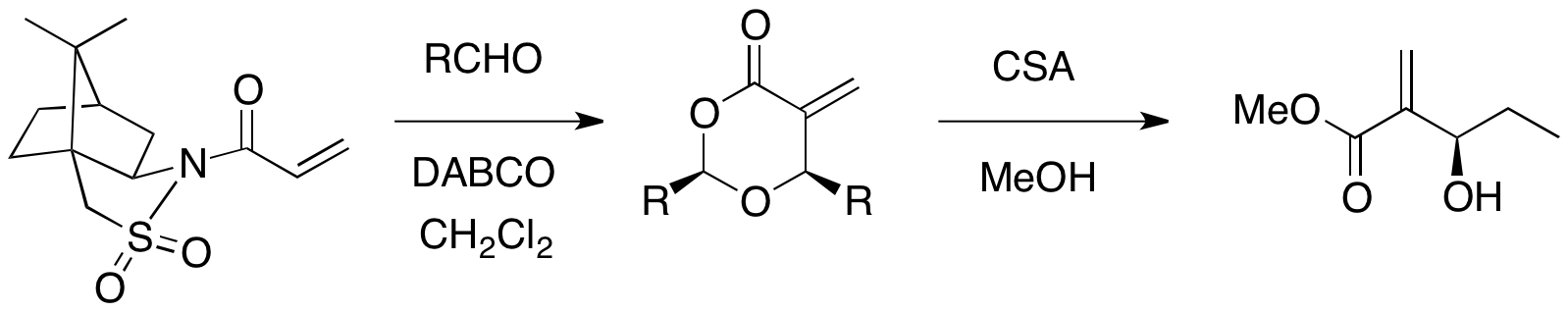
Oppolzer's sultam can be used as a chiral auxiliary for an asymmetric MBH reaction. When an acrylate substituted with the Oppolzer's sultam reacted with various aldehydes in the presence of DABCO catalyst, optically pure 1,3-dioxan-4-ones were afforded with cleavage of the auxiliary (67–98% yield, >99% ee). The cyclic products could be converted into desired MBH products by use ofcamphorsulfonic acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic ...
and methanol.
 A related hydrazide auxiliary is the chiral acryloylhydrazide, which reacts diastereoselectively with aldehydes. Both diastereomers could be obtained with different choice of solvents (DMSO vs. mixed THF and H2O), suggesting that the transition structure conformation is solvent-influenced.
Chiral allenes and imines can also be employed for an asymmetric DABCO-catalyzed ''aza''-MBH reaction. Optically active 10-phenylsulfonylisobornyl buta-2,3-dienoate reacts with an aryl imine to afford α-allenylamine in a diastereoselective manner (37–57% yield).
A related hydrazide auxiliary is the chiral acryloylhydrazide, which reacts diastereoselectively with aldehydes. Both diastereomers could be obtained with different choice of solvents (DMSO vs. mixed THF and H2O), suggesting that the transition structure conformation is solvent-influenced.
Chiral allenes and imines can also be employed for an asymmetric DABCO-catalyzed ''aza''-MBH reaction. Optically active 10-phenylsulfonylisobornyl buta-2,3-dienoate reacts with an aryl imine to afford α-allenylamine in a diastereoselective manner (37–57% yield).

Chiral Lewis-basic catalyst
Some enantioselective MBH reactions employ chiral tertiary amine catalysts. For example, β-ICD, a cinchona alkaloid derivative, is famous among thequinidine
Quinidine is a class I antiarrhythmic agent, class IA antiarrhythmic agent used to treat heart rhythm disturbances. It is a diastereomer of Antimalarial medication, antimalarial agent quinine, originally derived from the bark of the cinchona tre ...
framework-based catalysts, and catalyzed an enantioselective MBH reaction with 1,1,1,3,3,3,-hexafluoroisopropyl acrylate as the activated alkene:
 The phenolic oxygen of β-ICD was shown to be important in the reaction, implying that β-ICD acts as a Brønsted–Lowry acid, not just a nucleophile.
Cyclopentenone and various aromatic and aliphatic aldehydes undergo an asymmetric reaction using Fu's planar chiral DMAP catalyst in isopropanol (54–96% yield, 53–98% ee). In this case, magnesium iodide as a Lewis acid cocatalyst was required to accelerate the reaction.
The phenolic oxygen of β-ICD was shown to be important in the reaction, implying that β-ICD acts as a Brønsted–Lowry acid, not just a nucleophile.
Cyclopentenone and various aromatic and aliphatic aldehydes undergo an asymmetric reaction using Fu's planar chiral DMAP catalyst in isopropanol (54–96% yield, 53–98% ee). In this case, magnesium iodide as a Lewis acid cocatalyst was required to accelerate the reaction.
 ''P''-Chiral phosphines have been investigated.
Simple diamines can also be employed as MBH catalysts. Methyl vinyl ketone and various substituted benzaldehydes were found to undergo asymmetric MBH reaction. The chiral pyrrolidine catalyst was effective for ortho- and para-substituted electron-deficient benzaldehydes (75–99% yield, 8–73% ee).
''P''-Chiral phosphines have been investigated.
Simple diamines can also be employed as MBH catalysts. Methyl vinyl ketone and various substituted benzaldehydes were found to undergo asymmetric MBH reaction. The chiral pyrrolidine catalyst was effective for ortho- and para-substituted electron-deficient benzaldehydes (75–99% yield, 8–73% ee).
 Chiral phosphine MBH catalysts often contain Brønsted–Lowry acid moieties in their backbones. For example, chiral phosphines containing a Lewis base, a Brønsted–Lowry acid, and an acid-activated Brønsted–Lowry base were developed for an asymmetric ''aza''-MBH reaction (86–96% yield, 79–92% ee). The Brønsted–Lowry acid and base moieties were proposed to be involved in the stabilization of zwitterionic species in a stereoselective manner.
Chiral phosphine MBH catalysts often contain Brønsted–Lowry acid moieties in their backbones. For example, chiral phosphines containing a Lewis base, a Brønsted–Lowry acid, and an acid-activated Brønsted–Lowry base were developed for an asymmetric ''aza''-MBH reaction (86–96% yield, 79–92% ee). The Brønsted–Lowry acid and base moieties were proposed to be involved in the stabilization of zwitterionic species in a stereoselective manner.
 BINOL-derived chiral phosphine catalyst is also effective for an asymmetric aza-MBH reaction of N-tosyl imines with activated alkenes such as methyl vinyl ketone and phenyl acrylate.
In addition, a distinct class of chiral phosphine-
BINOL-derived chiral phosphine catalyst is also effective for an asymmetric aza-MBH reaction of N-tosyl imines with activated alkenes such as methyl vinyl ketone and phenyl acrylate.
In addition, a distinct class of chiral phosphine-squaramide
Squaramide is the organic compound with the formula O2C4(NH2)2. Not an amide in the usual sense, it is a derivative of squaric acid wherein the two OH groups are replaced by NH2 groups. Squaramides refer to a large class of derivatives wherein s ...
molecules could effectively catalyze an intramolecular asymmetric MBH reaction. ω-formylenones reacted to afford enantioenriched cyclic products at ambient temperature (64–98% yield, 88–93% ee).

Chiral Lewis acid catalyst
Chiral Lewis acid catalysts have been given interests as they could activate the electron-withdrawing group in an enantioselective manner. Chiral cationic oxazaborolidinium catalysts were shown to be effective in the three-component coupling of α,β-acetylenic esters, aldehydes, and trimethylsilyl iodide (50–99% yield, 62–94% ee). Both enantiomeric products could be obtained by using different enantiomers of the catalyst. Complex of metal salt and chiral ligand is a viable strategy, too. La(OTf)3 and camphor-derived chiral ligands could induce enantioselectivity in a DABCO-catalyzed MBH reaction of various aldehydes and acrylates (25–97% yield, 6–95% ee). For these cases, multidentate ligands were usually employed to chelate with the metal, which activates both the zwitterionic enolate and the aldehyde.
Complex of metal salt and chiral ligand is a viable strategy, too. La(OTf)3 and camphor-derived chiral ligands could induce enantioselectivity in a DABCO-catalyzed MBH reaction of various aldehydes and acrylates (25–97% yield, 6–95% ee). For these cases, multidentate ligands were usually employed to chelate with the metal, which activates both the zwitterionic enolate and the aldehyde.
 La(O-iPr)3 and BINOL-derived ligand system, in conjunction with catalytic DABCO, also works for an asymmetric aza-MBH reaction of various N-diphenylphosphinoyl imines and methyl acrylate. Aryl, heteroaryl, and alkenyl imines were all suitable for good yield and enantioselectivity.
Chiral palladium(II) pincer complexes function as Lewis acid in the enantioselective DABCO-catalyzed aza-MBH reaction of acrylonitrile and various tosyl imines to afford functionalized α-methylene-β-aminonitriles (75–98% yield, 76–98% ee). Silver acetate is required to activate the palladium bromide precatalyst in the catalytic cycle.
La(O-iPr)3 and BINOL-derived ligand system, in conjunction with catalytic DABCO, also works for an asymmetric aza-MBH reaction of various N-diphenylphosphinoyl imines and methyl acrylate. Aryl, heteroaryl, and alkenyl imines were all suitable for good yield and enantioselectivity.
Chiral palladium(II) pincer complexes function as Lewis acid in the enantioselective DABCO-catalyzed aza-MBH reaction of acrylonitrile and various tosyl imines to afford functionalized α-methylene-β-aminonitriles (75–98% yield, 76–98% ee). Silver acetate is required to activate the palladium bromide precatalyst in the catalytic cycle.

Chiral Brønsted–Lowry acid cocatalyst
A variety of chiralthiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), with the oxygen atom replaced by sulfur atom (as implied by the '' thio-'' prefix). The properties of urea and thiourea differ s ...
catalysts are under investigation for asymmetric MBH reactions. Chiral thiourea and bis(thiourea) catalysts can be effective in DABCO-catalyzed MBH and aza-MBH reactions. Jacobsen's thiourea catalyst performs an enantioselective aza-MBH reaction, for example (25–49% yield, 87–99% ee).
 While simple thiourea requires a nucleophilic catalyst in conjunction, bifunctional catalysts such as phosphine-thioureas can be used alone for asymmetric MBH reactions. For example, various acrylates and aromatic aldehydes react in the presence of these catalysts to afford either enantiomeric MBH adducts (32–96% yield, 9–77% ee).
While simple thiourea requires a nucleophilic catalyst in conjunction, bifunctional catalysts such as phosphine-thioureas can be used alone for asymmetric MBH reactions. For example, various acrylates and aromatic aldehydes react in the presence of these catalysts to afford either enantiomeric MBH adducts (32–96% yield, 9–77% ee).
 MBH reaction can involve proline derivative as a cocatalyst. It was proposed that imidazole nucleophilic catalyst and proline effect the reaction via iminium intermediate. With (S)-proline and DABCO, α-amido sulfones and α,β-unsaturated aldehydes undergo a highly enantioselective aza-MBH reaction (46–87% yield, E/Z 10:1–19:1, 82–99% ee).
MBH reaction can involve proline derivative as a cocatalyst. It was proposed that imidazole nucleophilic catalyst and proline effect the reaction via iminium intermediate. With (S)-proline and DABCO, α-amido sulfones and α,β-unsaturated aldehydes undergo a highly enantioselective aza-MBH reaction (46–87% yield, E/Z 10:1–19:1, 82–99% ee).

Applications in organic synthesis
The Baylis–Hillman adducts and their derivatives have been extensively utilized for the generation of heterocycles and other cyclic frameworks. MBH reactions are widely used in organic synthesis. For example, this reaction was used to construct key cyclic intermediates for syntheses of salinosporamide A, diversonol, and anatoxin-a.Chem. Commun. 2008, 3432.


Further reading
Many reviews have been written, including: * * G. Masson, C. Housseman and J. Zhu (2007), "The Enantioselective Morita–Baylis–Hillman Reaction and Its Aza Counterpart." ''Angewandte Chemie International Edition'', 46: 4614–4628. * Valerie Declerck, Jean Martinez and Frederic Lamaty (2009), "The ''aza''-Baylis−Hillman Reaction" ''Chem. Rev.'', 109 (1), pp. 1–48. * Deevi Basavaiah, Bhavanam Sekhara Reddy and Satpal Singh Badsara (2010), "Recent Contributions from the Baylis−Hillman Reaction to Organic Chemistry" ''Chemical Reviews'' 110 (9), pp. 5447–5674. * Javier Mansilla and José M. Saá (2010), "Enantioselective, Organocatalytic Morita-Baylis-Hillman and Aza-Morita-Baylis-Hillman Reactions: Stereochemical Issues." ''Molecules'' 15 (2), pp. 709–734. * Deevi Basavaiah and Gorre Veeraraghavaiah (2012), "The Baylis–Hillman reaction: a novel concept for creativity in chemistry" ''Chem. Soc. Rev.''References
{{DEFAULTSORT:Baylis-Hillman reaction Addition reactions Carbon-carbon bond forming reactions Name reactions