2,5-Dimethoxy-3,4-methylenedioxyamphetamine on:
[Wikipedia]
[Google]
[Amazon]
2,5-Dimethoxy-3,4-methylenedioxyamphetamine (DMMDA or DMMDA-1) is a lesser-known
 DMMDA is predicted to be
DMMDA is predicted to be
 Shulgin explains in his book that DMMDA has 6 isomers similar to TMA.
Shulgin explains in his book that DMMDA has 6 isomers similar to TMA.

DMMDA - Isomer Design
DMMDA - PiHKAL - Erowid
DMMDA - PiHKAL - Isomer Design
{{Phenethylamines 2,5-Dimethoxyphenethylamines Hydroxyquinol ethers Methylenedioxyphenethylamines Psychedelic phenethylamines
psychedelic drug
Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary mental states (known as psychedelic experiences or "trips") and a perceived "expansion of consciousness". Also referred to as classic halluc ...
of the amphetamine
Amphetamine (contracted from Alpha and beta carbon, alpha-methylphenethylamine, methylphenethylamine) is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, an ...
family related to MMDA. It was first synthesized by Alexander Shulgin
Alexander Theodore "Sasha" Shulgin (June 17, 1925 – June 2, 2014) was an American biochemist, broad researcher of synthetic psychoactive compounds, and author of works regarding these, who independently explored the organic chemistry and ph ...
in the 1960s and was described in his 1991 book ''PiHKAL
''PiHKAL: A Chemical Love Story'' is a book by Alexander Shulgin and Ann Shulgin published in 1991. The subject of the work is Psychoactive drug, psychoactive phenethylamine Derivative (chemistry), chemical derivatives, notably those that act ...
''.
Use and effects
Shulgin listed the dosage of DMMDA in ''PiHKAL'' as 30 to 75mg and the duration as 6 to 8hours. He reported DMMDA as producingLSD
Lysergic acid diethylamide, commonly known as LSD (from German ; often referred to as acid or lucy), is a semisynthetic, hallucinogenic compound derived from ergot, known for its powerful psychological effects and serotonergic activity. I ...
-like subjective effects: image
An image or picture is a visual representation. An image can be Two-dimensional space, two-dimensional, such as a drawing, painting, or photograph, or Three-dimensional space, three-dimensional, such as a carving or sculpture. Images may be di ...
s, mydriasis
Mydriasis is the Pupillary dilation, dilation of the pupil, usually having a non-physiological cause, or sometimes a physiological pupillary response. Non-physiological causes of mydriasis include disease, Physical trauma, trauma, or the use of c ...
, ataxia
Ataxia (from Greek α- negative prefix+ -τάξις rder= "lack of order") is a neurological sign consisting of lack of voluntary coordination of muscle movements that can include gait abnormality, speech changes, and abnormalities in e ...
, and time dilation
Time dilation is the difference in elapsed time as measured by two clocks, either because of a relative velocity between them (special relativity), or a difference in gravitational potential between their locations (general relativity). When unsp ...
. DMMDA is not mentioned much in literature outside ''PiHKAL''.
Pharmacology
Themechanism
Mechanism may refer to:
*Mechanism (economics), a set of rules for a game designed to achieve a certain outcome
**Mechanism design, the study of such mechanisms
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a ...
behind the subjective effects of DMMDA has not been specifically established. In ''PiHKAL'', Shulgin asserts that the subjective effects of 75mg of DMMDA are equivalent to those of 75 to 100μg of LSD
Lysergic acid diethylamide, commonly known as LSD (from German ; often referred to as acid or lucy), is a semisynthetic, hallucinogenic compound derived from ergot, known for its powerful psychological effects and serotonergic activity. I ...
. LSD is a well-known partial agonist
In pharmacology, partial agonists are drugs that bind to and activate a given Receptor (biochemistry), receptor, but have only partial Intrinsic activity, efficacy at the receptor relative to a full agonist. They may also be considered Ligand (bio ...
of the serotonin
Serotonin (), also known as 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter with a wide range of functions in both the central nervous system (CNS) and also peripheral tissues. It is involved in mood, cognition, reward, learning, ...
5-HT2A receptor. This may suggest that DMMDA is also an agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an R ...
or partial agonist of the 5-HT2A receptor.
DMMDA is equivalent to 12 "mescaline units" in terms of potency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of ho ...
. DMMDA's isomer DMMDA-2 is equivalent to 5 "mescaline units" in terms of potency.
Repeated administration of mescaline
Mescaline, also known as mescalin or mezcalin, and in chemical terms 3,4,5-trimethoxyphenethylamine, is a natural product, naturally occurring psychedelic drug, psychedelic alkaloid, protoalkaloid of the substituted phenethylamine class, found ...
(3,4,5-trimethoxyphenethylamine), a somewhat similar compound to DMMDA, has shown to slowly create tolerance. This may suggest that the same applies to DMMDA.
Computationally predicted toxicology and metabolism
Computational prediction with ProTox-3.0 predicts that DMMDA has the following toxicological properties from most probable to least probable: respiratorically toxic (P=0.76),nephrotoxic
Nephrotoxicity is toxicity in the kidneys. It is a poisonous effect of some substances, both toxic chemicals and medications, on kidney function. There are various forms, and some drugs may affect kidney function in more than one way. Nephrotoxin ...
(P=0.58), ecotoxic
Ecotoxicity, the subject of study in the field of ecotoxicology (a portmanteau of ecology and toxicology), refers to the biological, chemical or physical stressors that affect ecosystems. Such stressors can occur in the natural environment a ...
(P=0.54), and carcinogenic
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agent ...
(P=0.51). DMMDA is also predicted to cross the blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system ...
(BBB) (P=0.80).
:  DMMDA is predicted to be
DMMDA is predicted to be metabolized
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
via cytochrome CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by ''CYP3A4'' gene. It organic redox reaction, oxidizes small foreign organic molecules ( ...
(P=0.60). DMMDA is somewhat similar to 3,4-methylenedioxy-1-N,α-dimethylphenylethylamine which is primarily metabolized into 3,4-dihydroxy-1-N,α-dimethylphenylethylamine. This may suggest that DMMDA can be metabolized into 3,4-dihydroxy-2,5-dimethoxy-1-α-methylphenylethylamine. The metabolism of 3,4,5-trimethoxyphenylethylamine results in the demethylation of its methoxy groups, which may suggest that metabolism of DMMDA may also result in the demethylation of its methoxy groups.
Chemistry
 Shulgin explains in his book that DMMDA has 6 isomers similar to TMA.
Shulgin explains in his book that DMMDA has 6 isomers similar to TMA. DMMDA-2
DMMDA-2 is a bioactive phenethylamine discussed by Alexander Shulgin in his book '' PiHKAL (Phenethylamines i Have Known And Loved)''; however, he was not the first to synthesize it. Shulgin comments in his book that a 50 milligram dose of DMMDA ...
is the only other isomer that has been synthesized as of yet. DMMDA-3 could be made from exalatacin (1-allyl-2,6-dimethoxy-3,4-methylenedioxybenzene). Exalatacin can be found in the essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the ...
of both Crowea exalata and Crowea angustifolia var. angustifolia. In other words, exalatacin is an isomer of both apiole
Apiole is a phenylpropene, also known as apiol, parsley apiol, or parsley camphor. Its chemical name is 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene. It is found in the essential oils of celery leaf and all parts of parsley. Heinrich Christoph ...
and dillapiole
Dillapiole is an organic chemical compound and essential oil commonly extracted from dill weed, though it can be found in a variety of other plants such as fennel root. This compound is closely related to apiole, having a methoxy group positioned ...
, which can be used to make DMMDA and DMMDA-2 respectively. Additionally, yet another isomer of DMMDA could be made from pseudodillapiole or 4,5-dimethoxy-2,3-methylenedioxyallylbenzene. The last two isomers of DMMDA are 5,6-dimethoxy-2,3-methylenedioxy-1-α-methylphenylethylamine and 4,6-dimethoxy-2,3-methylenedioxy-1-α-methylphenylethylamine.
Like all other α-methylphenylethylamine derivative compounds, DMMDA and its regioisomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is a compound that contains the same number and type of atoms, but with a different connectivity (i.e. arrangement of bonds) between them. The ...
have two enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
s due to the methyl group being in the alpha position of the ethyl group in position number 1 on the benzene ring. There is no information regarding the differences in the pharmacological effects of (S)-DMMDA and (R)-DMMDA.
Shulgin's synthesis
Shulgin describes the synthesis of DMMDA fromapiole
Apiole is a phenylpropene, also known as apiol, parsley apiol, or parsley camphor. Its chemical name is 1-allyl-2,5-dimethoxy-3,4-methylenedioxybenzene. It is found in the essential oils of celery leaf and all parts of parsley. Heinrich Christoph ...
in his ''PiHKAL''. Apiole is subjected to an isomerization reaction to yield isoapiole by adding to solution of ethanolic potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
and holding the solution at a steam bath. Isoapiole is then nitrated via a Knoevenagel condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
A Knoevenagel condensation is a nucleophilic addition o ...
to 2-nitro-isoapiole or 1-(2,3-dimethoxy-3,4-methylenedioxyphenyl)-2-nitropropene by adding it to a stirred solution of acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
and pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
at ice-bath temperatures and treating the solution with tetranitromethane
Tetranitromethane or TNM is an organic oxidizer with chemical formula . Its chemical structure consists of four nitro groups attached to one carbon atom. In 1857 it was first synthesised by the reaction of sodium cyanoacetamide with nitric aci ...
. The pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
acts as a catalyst in this reaction. 2,5-dimethoxy-3,4-methylenedioxybenzaldehyde can also be used as precursors in this step of the synthesis. The 2-nitro-isoapiole is finally reduced to freebase DMMDA by adding it to a well-stirred and refluxing suspension of diethylether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
and lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthe ...
under an inert atmosphere. The reduction can also be achieved with pressurized hydrogen. Finally, the freebase DMMDA converted into its hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternati ...
salt.
: 
Other synthetic methods
Shulgin's synthesis of DMMDA can reasonably be considered unsafe, at least by modern standards, since it usestetranitromethane
Tetranitromethane or TNM is an organic oxidizer with chemical formula . Its chemical structure consists of four nitro groups attached to one carbon atom. In 1857 it was first synthesised by the reaction of sodium cyanoacetamide with nitric aci ...
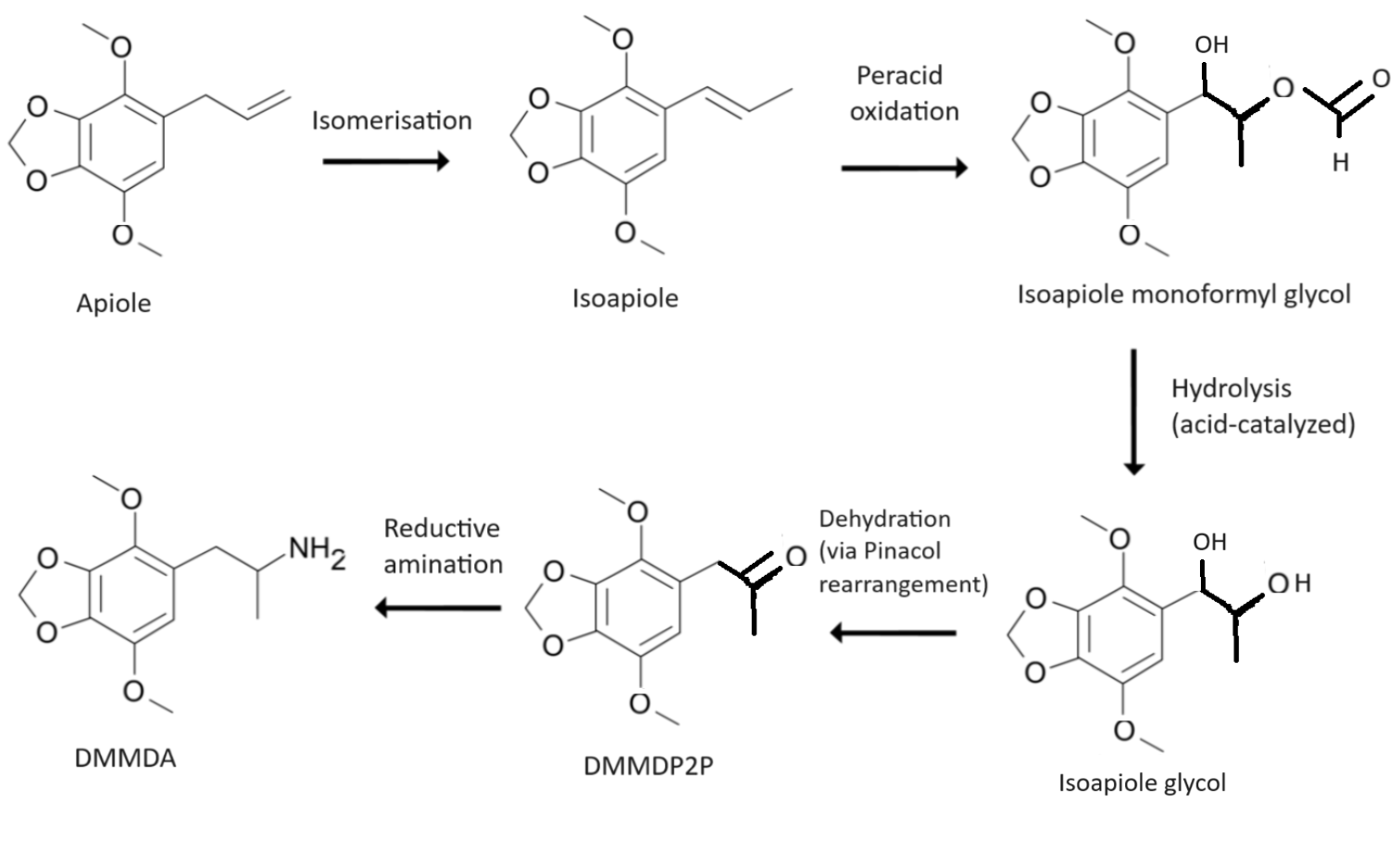
for its nitration reaction, which is toxic, carcinogenic and prone to detonating. DMMDA can be made from apiole via other safer methods. Among other methods, DMMDA can be synthesized from apiole via the intermediate chemical 2,5-dimethoxy-3,4-methylenedioxyphenylpropan-2-one or DMMDP2P in the same manner as MDA is made from safrole
Safrole is an organic compound with the formula CH2O2C6H3CH2CH=CH2. It is a colorless oily liquid, although impure samples can appear yellow. A member of the phenylpropanoid family of natural products, it is found in sassafras plants, among ot ...
.
DMMDP2P can be made from apiole via a Wacker oxidation with benzoquinone Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones:
* 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone)
* ...
. DMMDP2P can be alternatively made by subjecting apiole to an isomerisation
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeri ...
reaction to yield the thermodynamically stabler internal alkene, isoapiole, followed by a peroxyacid oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
, with for example peracetic acid, and finally a hydrolytic
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
dehydration
In physiology, dehydration is a lack of total body water that disrupts metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of water. Mild deh ...
. Peroxyacids can be made by combining hydrogen peroxide with an acid like formic acid or acetic acid to create performic acid or peracetic acid. It has been suggested that peroxynitric acid could also be used in this synthesis. The oxidation first creates an epoxide in the alkene of isoapiole and then isopaiole glycol's monoformate ester if peracetic acid is used. The hydrolysis is usually acid-catalyzed with a strong acid, such as sulphuric acid or hydrochloric acid, because the strong acid will also result in the intermediary isoapiole monoformyl glycol being dehydrated to DMMDP2P via a pinacol rearrangement. A small amount of the epoxide can form a carboxycation, which can rearrange itself to DMMDP2P, or react with water to form isoapiole glycol. Thus only one reagent, sulphuric acid, is needed for both the hydrolysis and dehydration and both reactions can be done in the same reaction vessel. Then the DMMDP2P can then be subjected to a reductive amination
Reductive amination (also known as reductive alkylation) is a form of amination that converts a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is a common method to make amine ...
with a source of nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, such as ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
or ammonium nitrate
Ammonium nitrate is a chemical compound with the formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, but does not form hydrates. It is predominantly us ...
, and a reducing agent, such as sodium cyanoborohydride
Sodium cyanoborohydride is a chemical compound with the formula . It is a colourless salt used in organic synthesis for chemical reduction including that of imines and carbonyls. Sodium cyanoborohydride is a milder reductant than other convention ...
, an amalgam
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
of mercury and aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
or pressurized hydrogen, to yield freebase DMMDA.
:
General synthetic information
Sodium borohydride usually is not used as a reducing agent due to it being much stronger than sodium cyanoborohydride; this usually results in side products in addition to DMMDA. Reductive aminations are exothermic reactions. Thus it is necessary to employ different methods of cooling the reaction mixture to prevent overheating; this can be accomplished by using a large amount of solvent or an ice bath, for example. The use of a mercury amalgam is unsafe due to mercury's well-known toxic effects on the central nervous system. In addition toperacetic acid
Peracetic acid (also known as peroxyacetic acid, or Percidine) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive.
Perac ...
, other peroxy acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy deri ...
s can be used for the peroxy acid oxidation of isoapiole and other analogues of isoallylbenzene in general. For example, combining nitric acid with hydrogen peroxide would result in the same reaction.
History
DMMDA was first described in thescientific literature
Scientific literature encompasses a vast body of academic papers that spans various disciplines within the natural and social sciences. It primarily consists of academic papers that present original empirical research and theoretical ...
by Alexander Shulgin
Alexander Theodore "Sasha" Shulgin (June 17, 1925 – June 2, 2014) was an American biochemist, broad researcher of synthetic psychoactive compounds, and author of works regarding these, who independently explored the organic chemistry and ph ...
and colleagues by 1967.
See also
*Substituted methylenedioxyphenethylamine
The substituted methylenedioxyphenethylamines (abbreviated as MDxx) represent a diverse chemical class of compounds derived from phenethylamines. This category encompasses numerous Psychoactive drug, psychoactive substances with Empathogen, entac ...
* Substituted methoxyphenethylamine
Methoxyphenethylamines (MPEAs), as well as methoxyamphetamines (MAs) in the case of the amphetamine (α-methylphenethylamine) homologues, are substituted phenethylamines with one or more methoxy groups. In some cases, one or more of the methoxy ...
* Dimethoxymethylenedioxyamphetamine
* Methoxymethylenedioxyamphetamine
* 2,3,4,5-Tetramethoxyamphetamine (TeMA)
References
External links
DMMDA - Isomer Design
DMMDA - PiHKAL - Erowid
DMMDA - PiHKAL - Isomer Design
{{Phenethylamines 2,5-Dimethoxyphenethylamines Hydroxyquinol ethers Methylenedioxyphenethylamines Psychedelic phenethylamines