2,2,4,4-Tetramethyl-1,3-cyclobutanediol on:
[Wikipedia]
[Google]
[Amazon]
2,2,4,4-Tetramethyl-1,3-cyclobutanediol (CBDO) is an aliphatic
diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting gro ...
. This diol is produced as a mixture of ''cis''- and ''trans''-isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s, depending on the relative stereochemistry of the hydroxyl groups. It is used as a monomer for the synthesis of polymeric materials, usually as an alternative to bisphenol A
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is Solubility, soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on a ...
(BPA). CBDO is used in the production of tritan copolyester which is used as a BPA-free replacement for polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...
.
Replacement for BPA
The controversies associated with BPA in large quantities are ultimately related to its endocrine disrupting abilities. Like BPA, CBDO is a diol with a structure suitable for making polyesters. CBDO’s C4 ring is sufficiently rigid to prevent the two OH groups from forming cyclic structures. Unlike BPA, there is no current evidence of carcinogenic or toxic effects from CBDO-based consumer products. There are, however, few studies on the toxicology of CBDO for both long term and short term effects. CBDO has potential advantages relative to BPA as a building block for production of polyesters. CBDO is very stable thermally and mechanically. Polyesters prepared from CBDO are rigid materials, but the combination of CBDO with flexible diols results in materials with high impact resistance, low color, thermal stability, good photooxidative stability and transparency. As an added bonus, CBDO-derived polymers have high ductility. The thermal and mechanical properties of CBDO-derived polyesters are often superior to conventional polyesters.Preparation
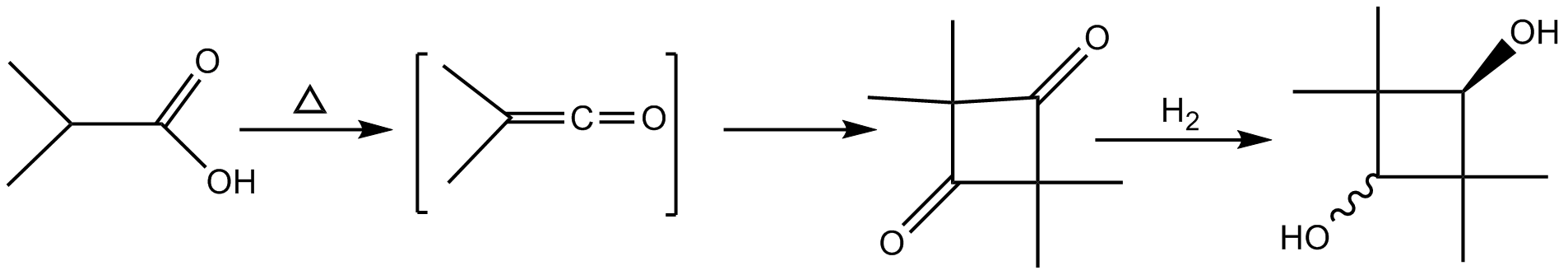
Synthesis of CBDO involves pyrolysis of isobutyric anhydride followed by hydrogenation of the resulting2,2,4,4-tetramethylcyclobutanedione
2,2,4,4-Tetramethylcyclobutanedione is the organic compound with the formula (CH3)4C4O2. The compound is a diketone of cyclobutane, bearing four methyl groups. It is a white solid that is used as a precursor to diverse industrial products.
Synth ...
. This synthesis resembles the method used to produce CBDO today. The first step involves conversion of the isobutyric acid
Isobutyric acid, also known as 2-methylpropanoic acid or isobutanoic acid, is a carboxylic acid with structural formula ( CH3)2CH COOH. It is an isomer of butyric acid. It is classified as a short-chain fatty acid. Deprotonation or esterificati ...
or its anhydride into the ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
. This ketene then dimerizes to form a four-membered ring with two ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
groups.
The product ring is hydrogenated to give a diol. The last step commonly involves catalytic hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
with ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chem ...
, nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
, or rhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
. Hydrogenation of the diketone ring results in both cis and trans isomers. A simplified scheme for the production of CBDO is presented below.

Structure and properties
The C4 ring of the cis isomer of CBDO is non-planar. For simple non-planar cyclobutanes, dihedral angles range from 19 to 31°. CBDO’s cis isomer crystallizes as two conformers with an average dihedral angle of 17.5° in the solid state. However, the trans isomer has a dihedral angle of 0°.Polyesterification
The current economic method for the production of polyesters is direct esterification of dicarboxylic acids with diols. This condensation polymerization adds monomeric units to a chain. Individual chains react with one another through carboxyl and hydroxyl terminal groups. Finally, transesterification occurs within the chain. Although CBDO is most often used in polyesters, mixed copolycarbonates of CBDO and a series of bisphenols have also been synthesized. The differing reactivities of the cis and trans isomers have not been studied in depth.References
{{DEFAULTSORT:Tetramethyl-1, 3-cyclobutanediol, 2, 2, 4, 4- Monomers Diols Cyclobutanes