|
2,2,4,4-Tetramethylcyclobutanedione
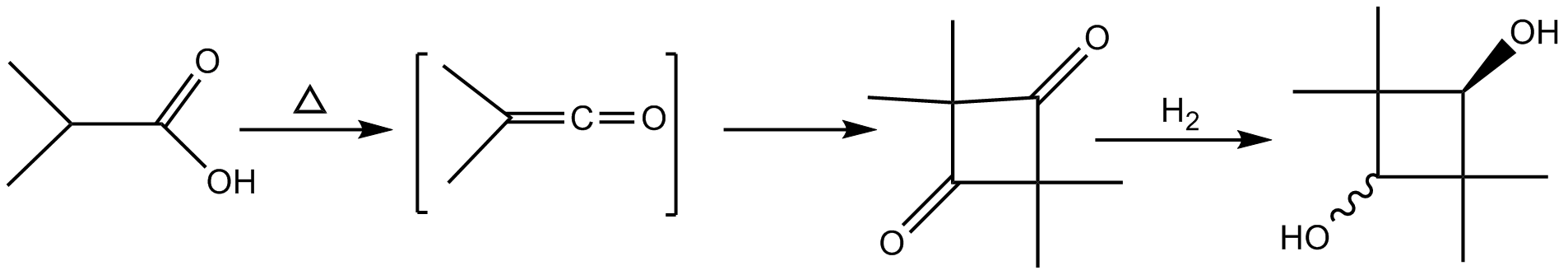
2,2,4,4-Tetramethylcyclobutanedione is the organic compound with the formula (CH3)4C4O2. The compound is a diketone of cyclobutane, bearing four methyl groups. It is a white solid that is used as a precursor to diverse industrial products. Synthesis and reactions 2,2,4,4-Tetramethylcyclobutanedione is the head-to-tail dimer of dimethylketene. It arises spontaneously when dimethylketene is produced by dehydrohalogenation of isobutyryl chloride with triethylamine. In the presence of aluminium trichloride, 2,2,4,4-tetramethylcyclobutanedione isomerizes to the lactone dimethylketene dimer (4-isopropylidene-3,3-dimethyl-2-oxetanone). Dimethylketene dimer is a precursor to various alkyl ketene dimers, which are used in papermaking. Hydrogenation Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl Ketene Dimer
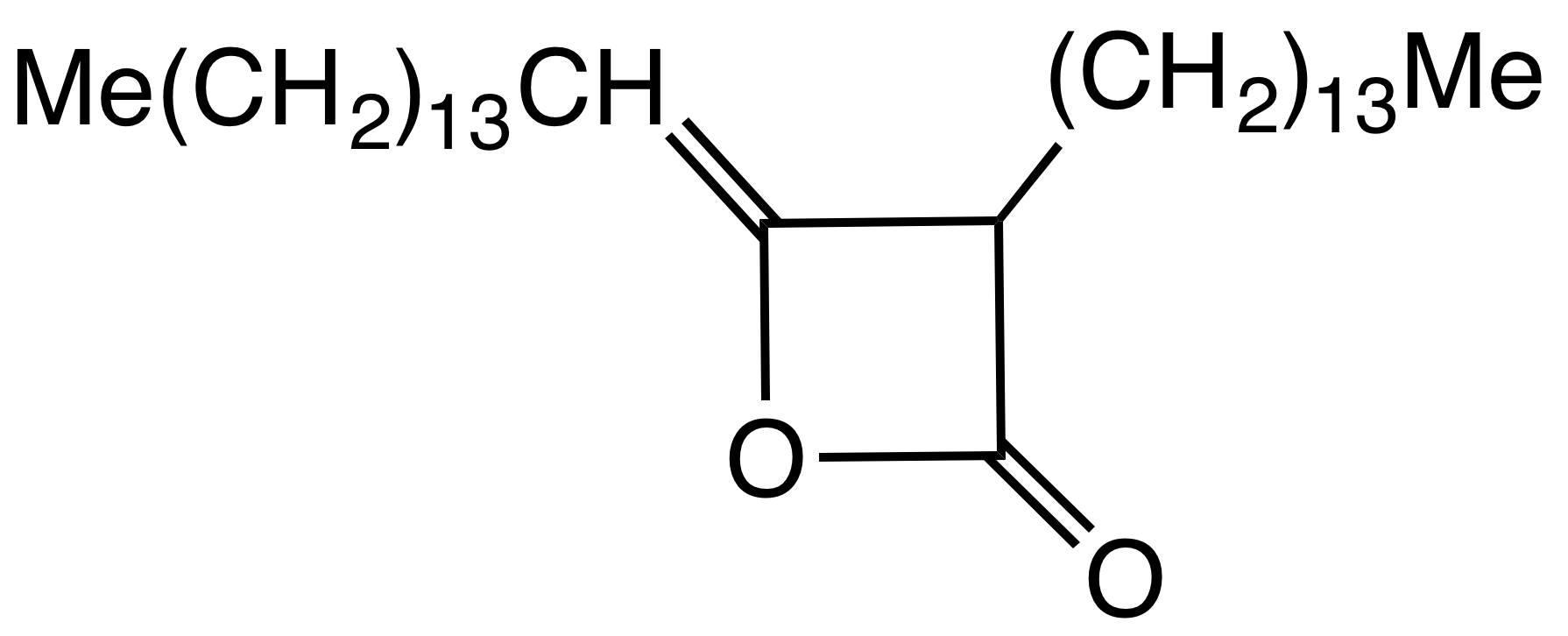
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of 2-oxetanone, oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position. The main application of alkylated ketene dimers is in the sizing of paper and cardboard, as well as in the hydrophobation of cellulose fibers. The products thus modified are distinguished by higher mechanical strengths and less penetration of water, inks or printing inks. AKD's feature hydrophobic alkyl groups extending from a beta-propiolactone ring. A specific example is derived from the dimerization of the ketene of stearic acid. This ketene is generated by pyrolysis of stearoyl chloride. AKD's react with the hydroxyl groups on the cellulose via esterification reaction. The esterification is competiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, diketones, diesters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydration is reversible. Glyoxal condenses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2,4,4-Tetramethyl-1,3-cyclobutanediol
2,2,4,4-Tetramethyl-1,3-cyclobutanediol (CBDO) is an aliphatic diol. This diol is produced as a mixture of ''cis''- and ''trans''-isomers, depending on the relative stereochemistry of the hydroxyl groups. It is used as a monomer for the synthesis of polymeric materials, usually as an alternative to bisphenol A (BPA). CBDO is used in the production of tritan copolyester which is used as a BPA-free replacement for polycarbonate. Replacement for BPA The controversies associated with BPA in large quantities are ultimately related to its endocrine disrupting abilities. Like BPA, CBDO is a diol with a structure suitable for making polyesters. CBDO’s C4 ring is sufficiently rigid to prevent the two OH groups from forming cyclic structures. Unlike BPA, there is no current evidence of carcinogenic or toxic effects from CBDO-based consumer products. There are, however, few studies on the toxicology of CBDO for both long term and short term effects. CBDO has potential advantages rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketones
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () Functional group, groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their Reactivity (chemistry), reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, diketones, diesters, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.''). 1,2-Dicarbonyls 1,2-Dialdehyde The only 1,2-dialdehyde is glyoxal, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehydes since hydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketene
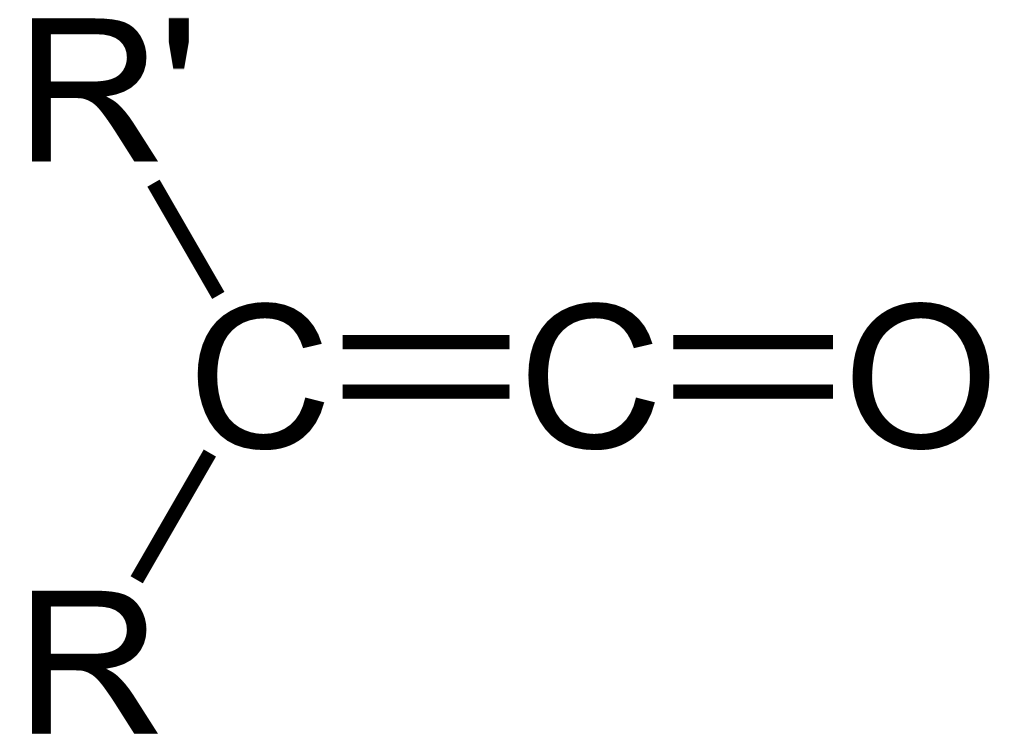
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutyryl Chloride
Isobutyryl chloride (2-methylpropanoyl chloride) is the organic compound with the formula . A colorless liquid, it the simplest branched-chain acyl chloride. It is prepared by chlorination of isobutyric acid. Reactions As an ordinary acid chloride, isobutyryl chloride is the subject of many reported transformations. Dehydrohalogenation of isobutyryl chloride with triethylamine gives 2,2,4,4-tetramethylcyclobutanedione. Treatment of isobutyryl chloride with hydrogen fluoride Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ... gives the acid fluoride. References Acyl chlorides Reagents for organic chemistry {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Trichloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour. The anhydrous form is commercially important. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |