|
Scavenger Receptor Cysteine-rich Domain
In molecular biology, the protein domain SRCR is short for Scavenger receptor cysteine-rich domain. They are found solely in eukaryotes. These domains are present on the cell membrane and have a role in binding to specific ligands and are often found to be involved with the immune system. Function The function of these endocytic receptors are to mediating non-opsonic phagocytosis in response to foreign ligands. This triggers various processes of host defence and immune response. Structure The structure contains a six stranded beta-sheet and one alpha-helix. Examples The speract receptor found in egg, is a transmembrane glycoprotein Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known a .... Other members of this family include the macrophage scavenger receptor type I, an enteropeptida ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Biology
Molecular biology is a branch of biology that seeks to understand the molecule, molecular basis of biological activity in and between Cell (biology), cells, including biomolecule, biomolecular synthesis, modification, mechanisms, and interactions. Though cells and other microscopic structures had been observed in living organisms as early as the 18th century, a detailed understanding of the mechanisms and interactions governing their behavior did not emerge until the 20th century, when technologies used in physics and chemistry had advanced sufficiently to permit their application in the biological sciences. The term 'molecular biology' was first used in 1945 by the English physicist William Astbury, who described it as an approach focused on discerning the underpinnings of biological phenomena—i.e. uncovering the physical and chemical structures and properties of biological molecules, as well as their interactions with other molecules and how these interactions explain observ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryotes
The eukaryotes ( ) constitute the domain of Eukaryota or Eukarya, organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of life forms alongside the two groups of prokaryotes: the Bacteria and the Archaea. Eukaryotes represent a small minority of the number of organisms, but given their generally much larger size, their collective global biomass is much larger than that of prokaryotes. The eukaryotes emerged within the archaeal kingdom Promethearchaeati and its sole phylum Promethearchaeota. This implies that there are only two domains of life, Bacteria and Archaea, with eukaryotes incorporated among the Archaea. Eukaryotes first emerged during the Paleoproterozoic, likely as flagellated cells. The leading evolutionary theory is they were created by symbiogenesis between an anaerobic Promethearchaeati archaean and an aerobic proteobacterium, which form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of a cell, being selectively permeable to ions and organic mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opson
''Opson'' () and ''sitos'' (σίτος) are an important division in Ancient Greek cuisine, Ancient Greek foodways. Opson is the 'relish' that complements the sitos; sitos is the staple food part of the meal, i.e. grains like wheat or barley, and pulses like chickpeas and fava beans. Although any kind of complement to the Staple food, staple, even salt, could be categorized as ''opson'', the term was also commonly used to refer to the most esteemed kind of relish: fish. Hence a diminutive of ''opson'', ''opsarion'' (ὀψάριον), provides the modern Greek word for fish: Medieval Greek, ''psari'' (ψάρι). ''Opson'' can also be used to mean 'prepared dish' (plural ''opsa''). Morality Because it was considered the more pleasurable part of any meal, ''opson'' was the subject of some anxiety among ancient Greek moralists, who coined the term ''opsophagos, opsophagia'' to describe the vice of those who took too much ''opson'' with their ''sitos''. The central focus of Greek pers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phagocytosis
Phagocytosis () is the process by which a cell (biology), cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte. In a Multicellular organism, multicellular organism's immune system, phagocytosis is a major mechanism used to remove pathogens and cell debris. The ingested material is then digested in the phagosome. Bacteria, dead tissue cells, and small mineral particles are all examples of objects that may be phagocytized. Some protozoa use phagocytosis as means to obtain nutrients. The two main cells that do this are the Macrophages and the Neutrophils of the immune system. Where phagocytosis is used as a means of feeding and provides the organism part or all of its nourishment, it is called phagotrophy and is distinguished from osmotrophy, which is nutrition taking place by absorption. History The history of phag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune Response
An immune response is a physiological reaction which occurs within an organism in the context of inflammation for the purpose of defending against exogenous factors. These include a wide variety of different toxins, viruses, intra- and extracellular bacteria, protozoa, Parasitic worm, helminths, and Fungus, fungi which could cause serious problems to the health of the host organism if not cleared from the body. In addition, there are other forms of immune response. For example, harmless exogenous factors (such as pollen and food components) can trigger allergy; latex and metals are also known allergens. A transplanted tissue (for example, blood) or organ can cause graft-versus-host disease. A type of immune reactivity known as Rh disease can be observed in pregnant women. These special forms of immune response are classified as hypersensitivity. Another special form of immune response is CD4+ T cells and antitumor immunity, antitumor immunity. In general, there are two branches of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common structural motif, motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone chain, backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of peptide, polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformational isomerism, conformation. The supramolecular association of β-sheets has been implicated in the formation of the Amyloid fibril, fibrils and Amyloid plaques, protein aggregates observed in amyloidosis, Alzheimer's disease and other Proteinopathy, proteinopathies. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix). The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of local structure, and it is the local structure that is most easily predicted from a sequence of amino acids. The alpha helix has a right-handed helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid that is four residues earlier in the protein sequence. Other names The alpha helix is also commonly called a: * Pauling–Corey–Branson α-helix (from the names of three scientists who described its structure) * 3.613-helix because there are 3.6 amino acids in one ring, with 13 atoms being involved in the ring formed by the hydrogen bond (starting with amidic hydrogen and ending with carbonyl oxygen) Discovery In the early 1930s, William Astbury showed that there were d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and Signal_transduction, transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses, such as a change in the electrophysiology, electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptor, GABA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand (biochemistry), ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Cell surface receptors, also known as transmembrane receptors, include ligand-gated ion channels, G prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
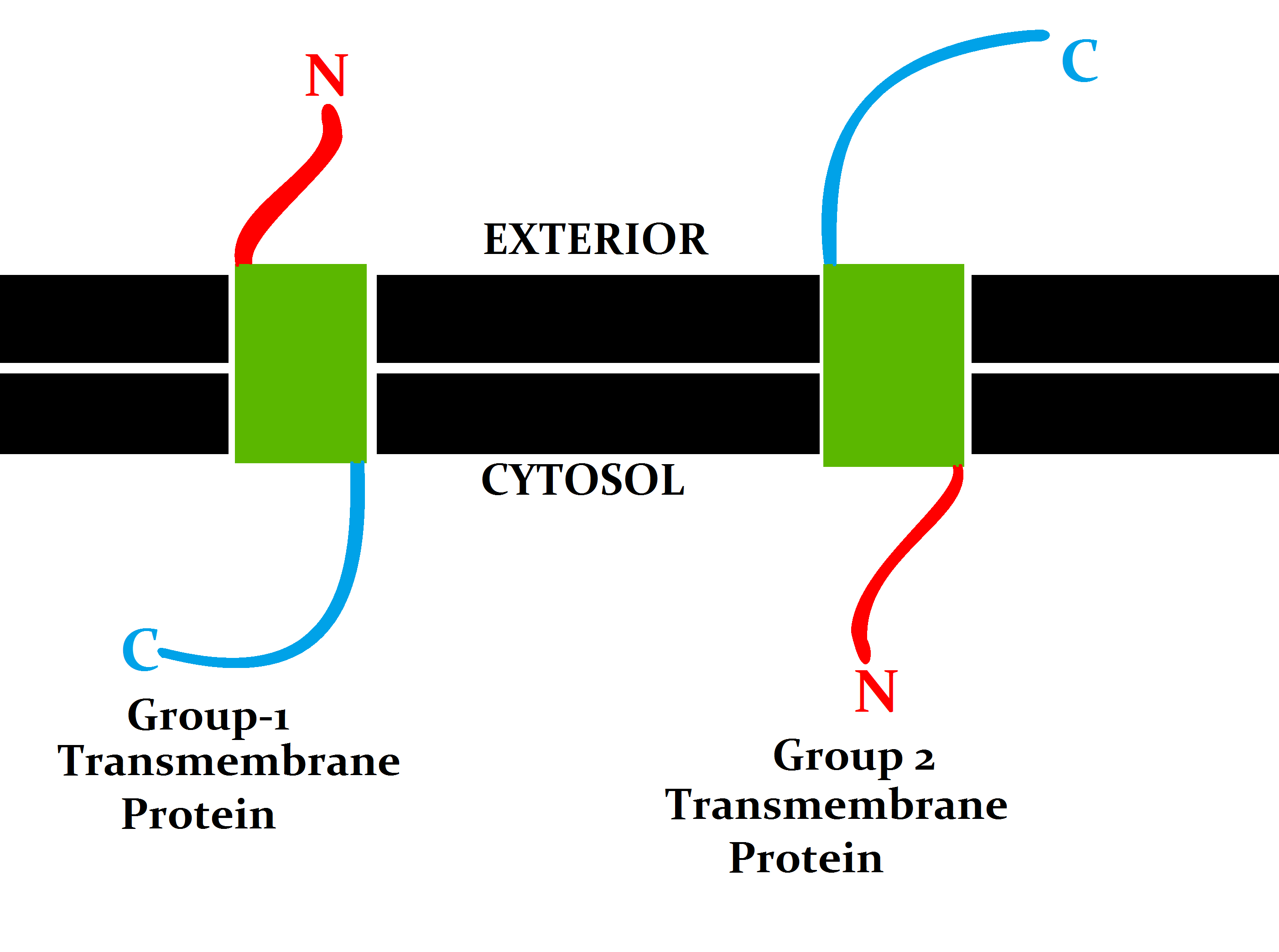
Transmembrane
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-pass membrane proteins, or as multipass membrane proteins. Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoprotein
Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |