|
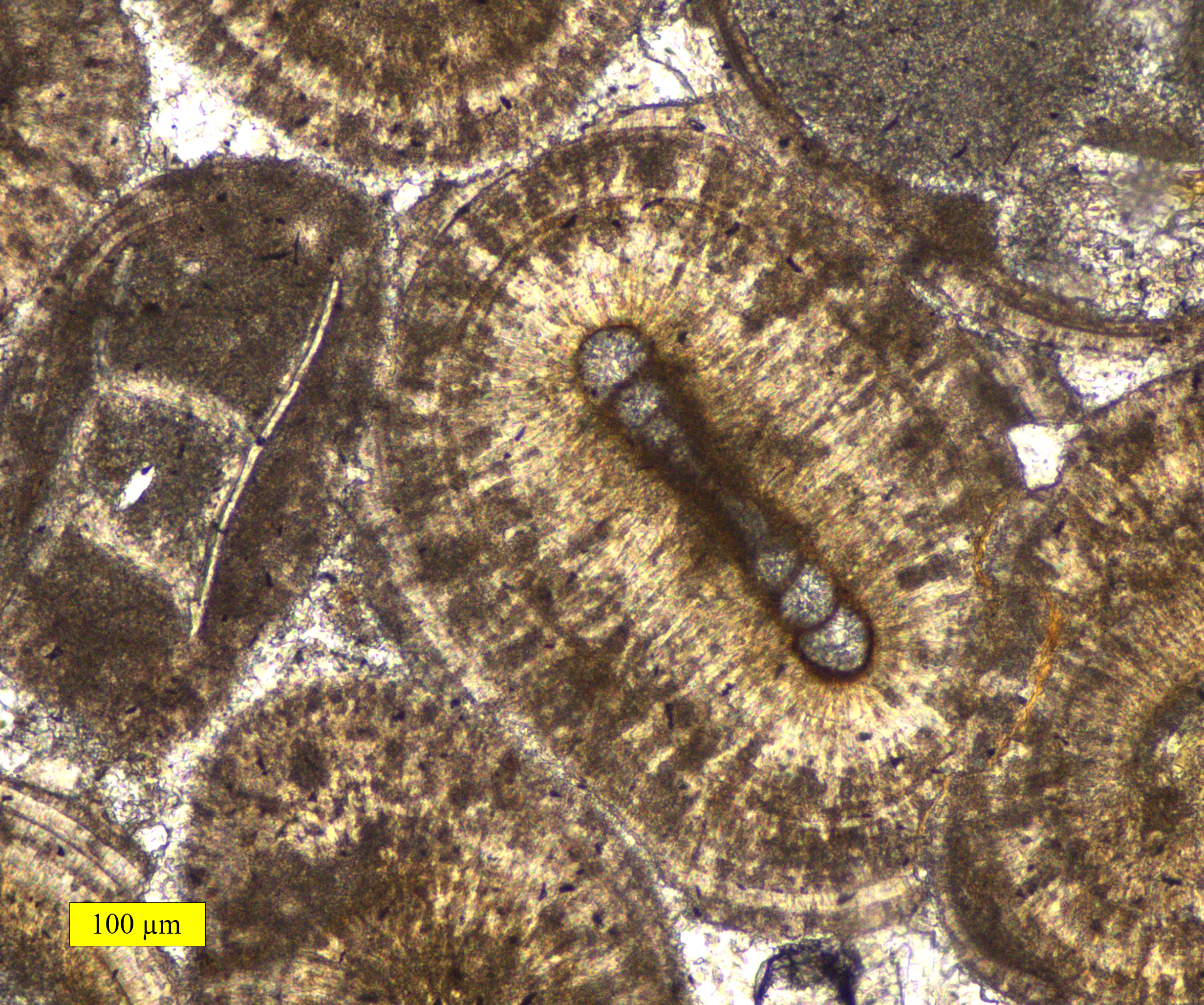
Oolith
Ooids (, ) are small (commonly ≤2 mm in diameter), spheroidal, "coated" (layered) sedimentary grains, usually composed of calcium carbonate, but sometimes made up of iron- or phosphate-based minerals. Ooids usually form on the sea floor, most commonly in shallow tropical seas (around the Bahamas, for example, or in the Persian Gulf). After being buried under additional sediment, these ooid grains can be cemented together to form a sedimentary rock called an ''oolite''. Oolites usually consist of calcium carbonate; these belong to the limestone rock family. Pisoids are similar to ooids, but are larger than 2 mm in diameter, often considerably larger, as with the pisoids in the hot springs at Carlsbad (Karlovy Vary) in the Czech Republic. Ooids have been the subject of scientific research for centuries.https://www.geological-digressions.com/the-mineralogy-of-carbonates-non-skeletal-grains/ Formation An ooid forms as a series of concentric layers around a nucleus. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jurassic
The Jurassic ( ) is a Geological period, geologic period and System (stratigraphy), stratigraphic system that spanned from the end of the Triassic Period million years ago (Mya) to the beginning of the Cretaceous Period, approximately 143.1 Mya. The Jurassic constitutes the second and middle period of the Mesozoic, Mesozoic Era as well as the eighth period of the Phanerozoic, Phanerozoic Eon and is named after the Jura Mountains, where limestone strata from the period were first identified. The start of the Jurassic was marked by the major Triassic–Jurassic extinction event, associated with the eruption of the Central Atlantic magmatic province, Central Atlantic Magmatic Province (CAMP). The beginning of the Toarcian Age started around 183 million years ago and is marked by the Toarcian Oceanic Anoxic Event, a global episode of Anoxic event, oceanic anoxia, ocean acidification, and elevated global temperatures associated with extinctions, likely caused by the eruption of the Kar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oolite
Oolite or oölite () is a sedimentary rock formed from ooids, spherical grains composed of concentric layers. Strictly, oolites consist of ooids of diameter 0.25–2 millimetres; rocks composed of ooids larger than 2 mm are called pisolites. The term ''oolith'' can refer to oolite or individual ooids. Composition Ooids are most commonly composed of calcium carbonate (calcite or aragonite), but can be composed of phosphate, clays, chert, dolomite or iron minerals, including hematite. Dolomitic and chert ooids are most likely the result of the replacement of the original texture in limestone. Oolitic hematite occurs at Red Mountain near Birmingham, Alabama, along with oolitic limestone. They are usually formed in warm, supersaturated, shallow, highly agitated marine water intertidal environments, though some are formed in inland lakes. The mechanism of formation starts with a small fragment of sediment acting as a 'seed', such as a piece of a shell. Strong intertidal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science), crystal forms of calcium carbonate . Limestone forms when these minerals Precipitation (chemistry), precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly Dolomite (rock), dolomite, a closely related rock, which contains a high percentage of the mineral Dolomite (mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of aragoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ordovician
The Ordovician ( ) is a geologic period and System (geology), system, the second of six periods of the Paleozoic Era (geology), Era, and the second of twelve periods of the Phanerozoic Eon (geology), Eon. The Ordovician spans 41.6 million years from the end of the Cambrian Period Megaannum, Ma (million years ago) to the start of the Silurian Period Ma. The Ordovician, named after the Celtic Britons, Welsh tribe of the Ordovices, was defined by Charles Lapworth in 1879 to resolve a dispute between followers of Adam Sedgwick and Roderick Murchison, who were placing the same Rock (geology), rock beds in North Wales in the Cambrian and Silurian systems, respectively. Lapworth recognized that the fossil fauna in the disputed Stratum, strata were different from those of either the Cambrian or the Silurian systems, and placed them in a system of their own. The Ordovician received international approval in 1960 (forty years after Lapworth's death), when it was adopted as an official per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite Sea
A calcite sea is a sea in which low-magnesium calcite is the primary inorganic marine calcium carbonate precipitate. An aragonite sea is the alternate seawater chemistry in which aragonite and high-magnesium calcite are the primary inorganic carbonate precipitates. The Early Paleozoic and the Middle to Late Mesozoic oceans were predominantly calcite seas, whereas the Middle Paleozoic through the Early Mesozoic and the Cenozoic (including today) are characterized by aragonite seas. The most significant geological and biological effects of calcite sea conditions include rapid and widespread formation of carbonate hardgrounds, calcitic ooids, calcite cements, and the contemporaneous dissolution of aragonite shells in shallow warm seas. Hardgrounds were very common, for example, in the calcite seas of the Ordovician and Jurassic, but virtually absent from the aragonite seas of the Permian. Fossils of invertebrate organisms found in calcite sea deposits are usually dominated by eit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
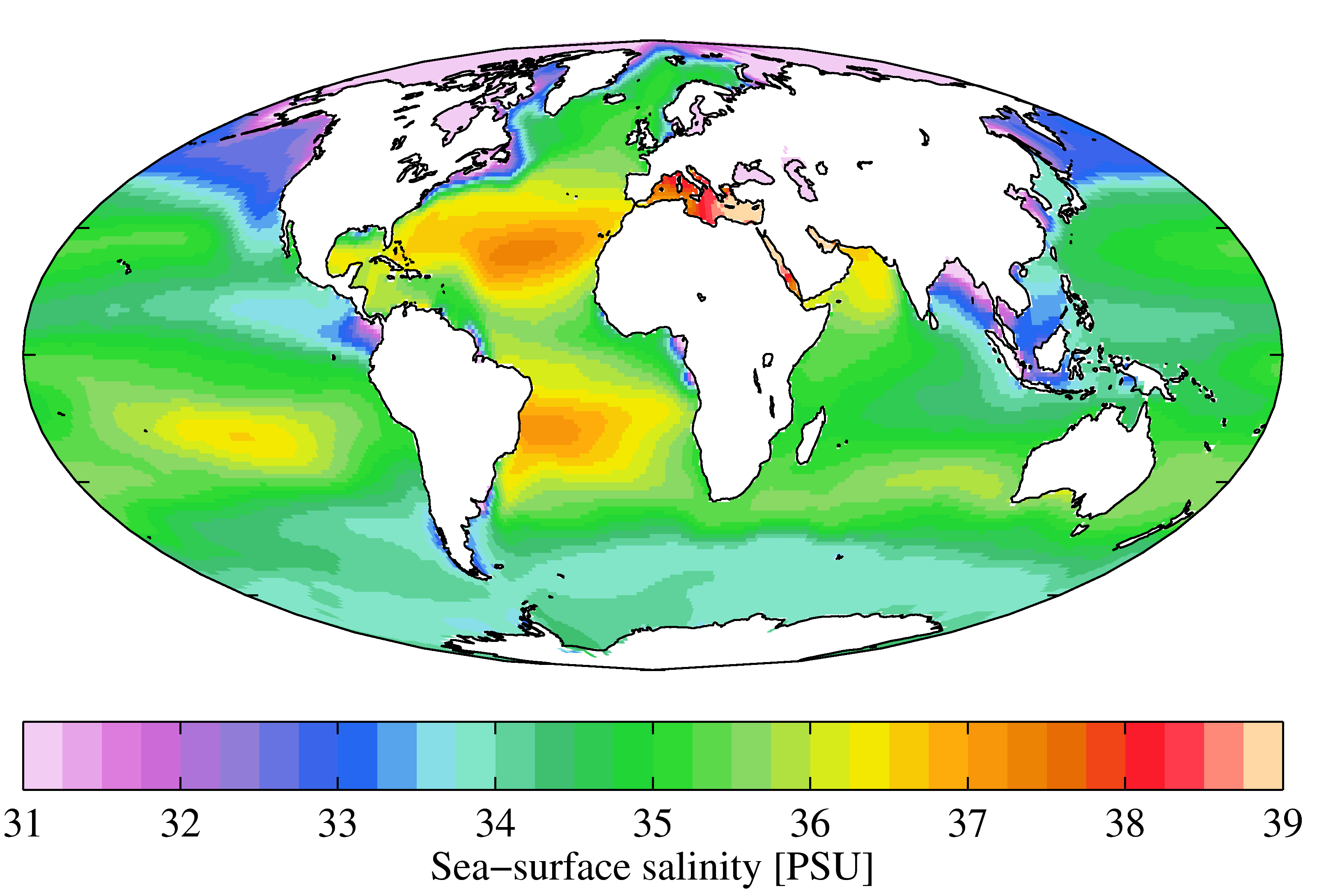
Salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to per mille, ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a state function, thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marine Environment
A marine habitat is a habitat that supports marine life. Marine life depends in some way on the saltwater that is in the sea (the term ''marine'' comes from the Latin ''mare'', meaning sea or ocean). A habitat is an ecological or environmental area inhabited by one or more living species.Abercrombie, M., Hickman, C.J. and Johnson, M.L. 1966.''A Dictionary of Biology.'' Penguin Reference Books, London The marine environment supports many kinds of these habitats. Marine habitats can be divided into coastal and open ocean habitats. Coastal habitats are found in the area that extends from as far as the tide comes in on the shoreline out to the edge of the continental shelf. Most marine life is found in coastal habitats, even though the shelf area occupies only seven percent of the total ocean area. Open ocean habitats are found in the deep ocean beyond the edge of the continental shelf. Alternatively, marine habitats can be divided into pelagic and demersal zones. Pelagic ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
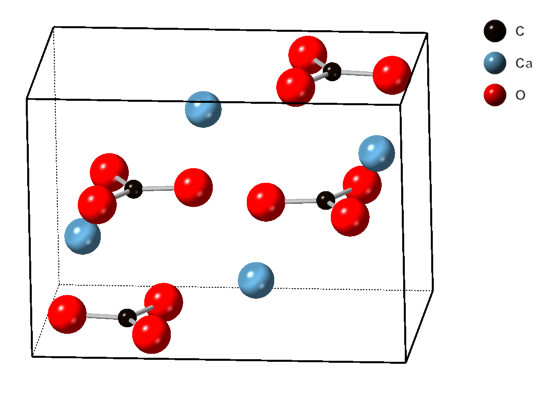
Crystalline Structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in a material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of principal axes/edges, of the unit cell and angles between them are lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of a crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see strontian proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |