|
Naphthalen-1,8-diyl 1,3,2,4-dithiadiphosphetane 2,4-disulfide
NpP2S4 is a compound related to Lawesson's reagent formed by the reaction of 1- bromonaphthalene with P4S10, this is a 1,3,2,4-dithiadiphosphetane 2,4-disulfide which has a naphth-1,8-diyl group holding the two phosphorus atoms together. The mechanism by which the NpP2S4 forms is not yet elucidated, but it is thought to occur by a process involving free radicals, and naphthalene has been detected as a side product in its synthesis. In general, NpP2S4 has been found to be less reactive than Lawesson's reagent, in agreement with the hypothesis that the dithiophosphine ylides are responsible for the majority of the chemical reactions of the 1,3,2,4-dithiadiphosphetane 2,4-disulfides. NpP2S4 has been found to react with many hydroxyl compounds, such as methanol, ethylene glycol and a catechol to form species with oxygen Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lawesson's Reagent
Lawesson's reagent (LR) is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10. Preparation Lawesson's reagent is commercially available. It can also be conveniently prepared in the laboratory by heating a mixture of anisole with phosphorus pentasulfide until the mixture is clear and no more hydrogen sulfide is formed, then recrystallized from toluene or xylene. Samples give a strong odor of hydrogen sulfide owing to partial hydrolysis. One common and effective method of destroying the foul smelling residues is to use an excess of sodium hypochlorite ( chlorine bleach). Mechanism of action Lawesson's reagent has a four membered ring of alternating sulfur and phosphorus atoms. The central phosphorus/sulfur four-membered ring dissociates to form two reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. Ethylene glycol has a sweet taste, but it is toxic in high concentrations. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation: This reaction can be catalyzed by either acids or bases, or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetraethylene glycol. The separation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagents For Organic Chemistry
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, '' catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Catechol occurs as feathery white crystals that are very rapidly soluble in water. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' ('' Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Bromonaphthalene
1-Bromonaphthalene is an organic compound with the formula C10H7Br. It is one of two isomeric bromonaphthalenes, the other being 2-bromonaphthalene. Under normal conditions, the substance is a colorless liquid. Synthesis and reactions It is prepared by treatment of naphthalene Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ... with bromine: :C10H8 + Br2 → C10H7Br + HBr The compound exhibits many reactions typical of aryl bromides. Bromide can be displaced by cyanide to give the nitrile. It forms a Grignard reagent and organolithium compound. 1-Lithionaphthalene can be further lithiated to give 1,8-dilithionaphthalene, a precursor to peri-naphthalene compounds. Applications Because of its high refractive index, (1.656-1.659nD) 1-bromonaphthalene is used as an embedd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
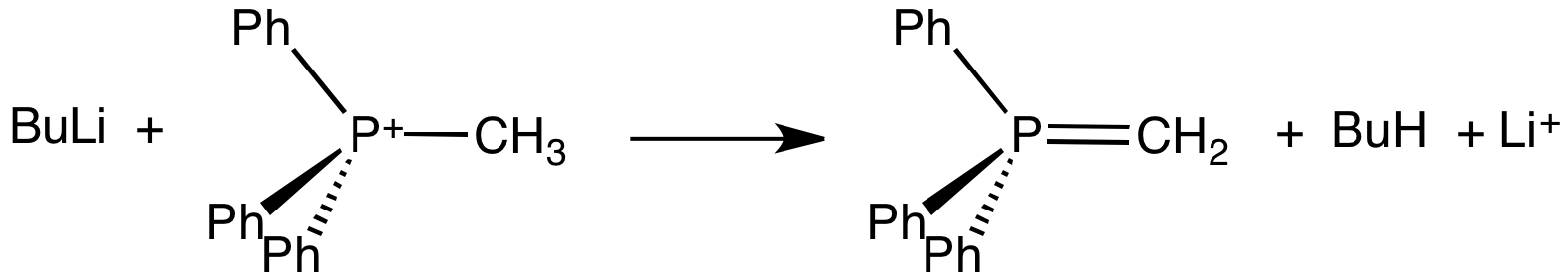
Ylide
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2- dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction betw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypothesis
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can testable, test it. Scientists generally base scientific hypotheses on previous observations that cannot satisfactorily be explained with the available scientific theories. Even though the words "hypothesis" and "theory" are often used interchangeably, a scientific hypothesis is not the same as a scientific theory. A working hypothesis is a provisionally accepted hypothesis proposed for further research in a process beginning with an educated guess or thought. A different meaning of the term ''hypothesis'' is used in formal logic, to denote the antecedent (logic), antecedent of a proposition; thus in the proposition "If ''P'', then ''Q''", ''P'' denotes the hypothesis (or antecedent); ''Q'' can be called a consequent. ''P'' is the :wikt:assumption, assumption in a (possibly Counterfactual conditional, counterfac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |