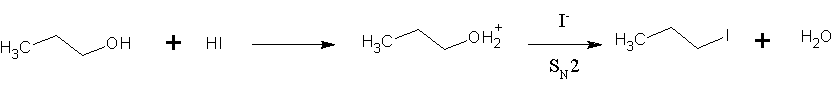|
Iodosilane
Iodosilane is a chemical compound of silicon, hydrogen, and iodine. It is a colorless monoclinic crystal of space group P21/c at −157 °C. Preparation Iodosilane is the first product of the reaction between monosilane and iodine, the other products being di-, tri- and finally tetraiodosilane ( silicon tetraiodide). It can also produced by the reaction of phenylsilane Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6 H5 SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have ... or chlorophenylsilane with hydrogen iodide. :\mathrm Properties At low temperatures, iodosilant quickly reacts with o(CO)4sup>− to form SiH3Co(CO)4. Further reading * * * References Silanes Iodides Nonmetal halides {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Tetraiodide
Silicon tetraiodide is the chemical compound with the formula Si I4. It is a tetrahedral molecule with Si-I bond lengths of 2.432(5) Å. SiI4 is a precursor to silicon amides of the formula Si(NR2)4 (R = alkyl). It has also been of interest in the manufacture and etching of silicon in microelectronics. Reactions This compound is stable among strong heating. It can be stored at room temperature for long periods but must be kept dry because it reacts quickly with water and moisture in the air. It can be made on a large scale by reaction of silicon or silicon carbide with iodine on heating to about 200 °C. Of more academic interest is the reaction of silane with iodine vapour at 130 - 150 °C, as this produces a series of compounds ranging from iodosilane SiH3I to diiodosilane SiH2I2 and triiodosilane SiHI3 as well. These compounds are colourless liquids at room temperature. The last one can be readily distinguished from the similar carbon compound, iodoform Iodoform (also k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal classes The table below org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monosilane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : Mg2Si + 4 HCl -> 2 MgCl2 + SiH4 It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : Si + 3 HCl -> HSiCl3 + H2 The trichlorosilane is then converted to a mixture of silane and silicon tetrachloride: : 4 HSi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylsilane
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6 H5 SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have similar densities and boiling points due to these similarities. Phenylsilane is soluble in organic solvents. Synthesis and reactions Phenylsilane is produced in two steps from Si(OEt)4. In the first step, phenylmagnesium bromide is added to form Ph−Si(OEt)3 via a Grignard reaction. Reduction of the resulting Ph−Si(OEt)3 product with LiAlH4 affords phenylsilane. :Ph−MgBr + Si(OEt)4 → Ph−Si(OEt)3 + MgBr(OEt) :4 Ph−Si(OEt)3 + 3 LiAlH4 → 4 Ph−SiH3 + 3 LiAl(OEt)4 Uses Phenylsilane can be used to reduce tertiary phosphine oxides to the corresponding tertiary phosphine. :P(CH3)3O + PhSiH3 → P(CH3)3 + PhSiH2OH The use of phenylsilane proceeds with retention of configuration at the phosphine. For example, cyclic chiral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent. Properties of hydrogen iodide HI is a colorless gas that reacts with oxygen to give water and iodine. With moist air, HI gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water, giving hydroiodic acid. One liter of water will dissolve 425 liters of HI gas, the most concentrated solution having only four water molecules per molecule of HI. Hydroiodic acid Hydroiodic acid is not pure hydrogen iodide, but a mixture containing it. Commercial "concentrated" hydroiodic acid usually contains 48–57% HI by mass. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |