|
Hard Spheres
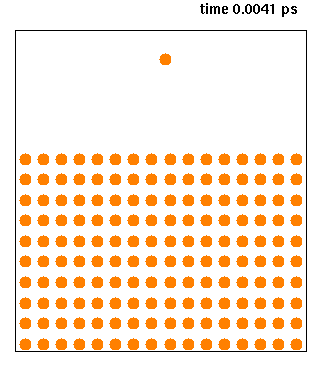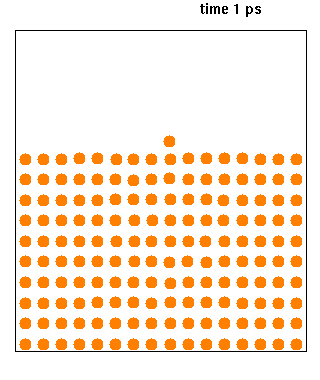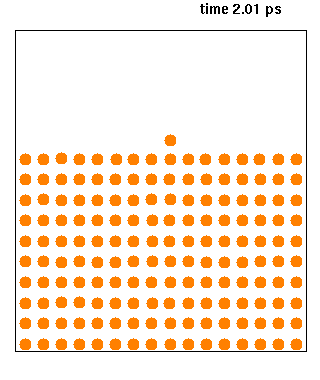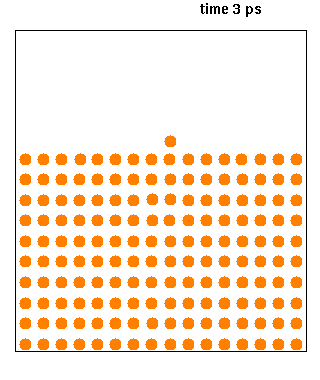
Hard spheres are widely used as model particles in the statistical mechanical theory of fluids and solids. They are defined simply as impenetrable spheres that cannot overlap in space. They mimic the extremely strong ("infinitely elastic bouncing") repulsion that atoms and spherical molecules experience at very close distances. Hard spheres systems are studied by analytical means, by molecular dynamics simulations, and by the experimental study of certain colloidal model systems. The hard-sphere system provides a generic model that explains the quasiuniversal structure and dynamics of simple liquids. Formal definition Hard spheres of diameter \sigma are particles with the following pairwise interaction potential: :V(\mathbf_1,\mathbf_2)=\left\{ \begin{matrix}0 & \mbox{if}\quad , \mathbf{r}_1-\mathbf{r}_2, \geq \sigma \\ \infty & \mbox{if}\quad, \mathbf{r}_1-\mathbf{r}_2, < \sigma \end{matrix} \right. where and are th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. It does not assume or postulate any natural laws, but explains the macroscopic behavior of nature from the behavior of such ensembles. Statistical mechanics arose out of the development of classical thermodynamics, a field for which it was successful in explaining macroscopic physical properties—such as temperature, pressure, and heat capacity—in terms of microscopic parameters that fluctuate about average values and are characterized by probability distributions. This established the fields of statistical thermodynamics and statistical physics. The founding of the field of statistical mechanics is generally credited to three physicists: * Ludwig Boltzmann, who developed the fundamental interpretation of entropy in terms of a collection of microstates *James Clerk Maxwell, who developed models of probability di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the system. In the most common version, the trajectories of atoms and molecules are determined by numerically solving Newton's equations of motion for a system of interacting particles, where forces between the particles and their potential energies are often calculated using interatomic potentials or molecular mechanical force fields. The method is applied mostly in chemical physics, materials science, and biophysics. Because molecular systems typically consist of a vast number of particles, it is impossible to determine the properties of such complex systems analytically; MD simulation circumvents this problem by using numerical methods. However, long MD simulations are mathematically ill-conditioned, generating cumulative erro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word '' suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study was introduced in 1845 by Ital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Virial Coefficient
Virial coefficients B_i appear as coefficients in the virial expansion of the pressure of a many-particle system in powers of the density, providing systematic corrections to the ideal gas law. They are characteristic of the interaction potential between the particles and in general depend on the temperature. The second virial coefficient B_2 depends only on the pair interaction between the particles, the third (B_3) depends on 2- and non-additive 3-body interactions, and so on. Derivation The first step in obtaining a closed expression for virial coefficients is a cluster expansion of the grand canonical partition function : \Xi = \sum_ = e^ Here p is the pressure, V is the volume of the vessel containing the particles, k_B is Boltzmann's constant, T is the absolute temperature, \lambda =\exp mu/(k_BT) is the fugacity, with \mu the chemical potential. The quantity Q_n is the canonical partition function of a subsystem of n particles: : Q_n = \operatorname e^ Here H(1,2, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monte Carlo Integration
In mathematics, Monte Carlo integration is a technique for numerical integration using random numbers. It is a particular Monte Carlo method that numerically computes a definite integral. While other algorithms usually evaluate the integrand at a regular grid, Monte Carlo randomly chooses points at which the integrand is evaluated. This method is particularly useful for higher-dimensional integrals. There are different methods to perform a Monte Carlo integration, such as uniform sampling, stratified sampling, importance sampling, sequential Monte Carlo (also known as a particle filter), and mean-field particle methods. Overview In numerical integration, methods such as the trapezoidal rule use a deterministic approach. Monte Carlo integration, on the other hand, employs a non-deterministic approach: each realization provides a different outcome. In Monte Carlo, the final outcome is an approximation of the correct value with respective error bars, and the correct valu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Packing Density
A packing density or packing fraction of a packing in some space is the fraction of the space filled by the figures making up the packing. In simplest terms, this is the ratio of the volume of bodies in a space to the volume of the space itself. In packing problems, the objective is usually to obtain a packing of the greatest possible density. In compact spaces If are measurable subsets of a compact measure space and their interiors pairwise do not intersect, then the collection is a packing in and its packing density is :\eta = \frac. In Euclidean space If the space being packed is infinite in measure, such as Euclidean space, it is customary to define the density as the limit of densities exhibited in balls of larger and larger radii. If is the ball of radius centered at the origin, then the density of a packing is :\eta = \lim_\frac. Since this limit does not always exist, it is also useful to define the upper and lower densities as the limit superior and limit i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Random Close Pack
Random close packing (RCP) of spheres is an empirical parameter used to characterize the maximum volume fraction of solid objects obtained when they are packed randomly. For example, when a solid container is filled with grain, shaking the container will reduce the volume taken up by the objects, thus allowing more grain to be added to the container. In other words, shaking increases the density of packed objects. But shaking cannot increase the density indefinitely, a limit is reached, and if this is reached without obvious packing into an ordered structure, such as a regular crystal lattice, this is the empirical random close-packed density for this particular procedure of packing. The random close packing is the highest possible volume fraction out of all possible packing procedures. Experiments and computer simulations have shown that the most compact way to pack hard perfect same-size spheres randomly gives a maximum volume fraction of about 64%, i.e., approximately 64% of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Close-packing Of Equal Spheres
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a lattice packing is :\frac \approx 0.74048. The same packing density can also be achieved by alternate stackings of the same close-packed planes of spheres, including structures that are aperiodic in the stacking direction. The Kepler conjecture states that this is the highest density that can be achieved by any arrangement of spheres, either regular or irregular. This conjecture was proven by T. C. Hales. Highest density is known only for 1, 2, 3, 8, and 24 dimensions. Many crystal structures are based on a close-packing of a single kind of atom, or a close-packing of large ions with smaller ions filling the spaces between them. The cubic and hexagonal arrangements are very close to one anoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Static Structure Factor
In condensed matter physics and crystallography, the static structure factor (or structure factor for short) is a mathematical description of how a material scatters incident radiation. The structure factor is a critical tool in the interpretation of scattering patterns (interference patterns) obtained in X-ray, electron and neutron diffraction experiments. Confusingly, there are two different mathematical expressions in use, both called 'structure factor'. One is usually written S(\mathbf); it is more generally valid, and relates the observed diffracted intensity per atom to that produced by a single scattering unit. The other is usually written F or F_ and is only valid for systems with long-range positional order — crystals. This expression relates the amplitude and phase of the beam diffracted by the (hk\ell) planes of the crystal ((hk\ell) are the Miller indices of the planes) to that produced by a single scattering unit at the vertices of the primitive unit cell. F_ is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Percus–Yevick Approximation
In statistical mechanics the Percus–Yevick approximation is a closure relation to solve the Ornstein–Zernike equation. It is also referred to as the Percus–Yevick equation. It is commonly used in fluid theory to obtain e.g. expressions for the radial distribution function. The approximation is named after Jerome K. Percus and George J. Yevick. Derivation The direct correlation function represents the direct correlation between two particles in a system containing ''N'' − 2 other particles. It can be represented by : c(r)=g_(r) - g_(r) \, where g_(r) is the radial distribution function, i.e. g(r)=\exp \beta w(r)/math> (with ''w''(''r'') the potential of mean force) and g_(r) is the radial distribution function without the direct interaction between pairs u(r) included; i.e. we write g_(r)=\exp \beta(w(r)-u(r))/math>. Thus we ''approximate'' ''c''(''r'') by : c(r)=e^- e^. \, If we introduce the function y(r)=e^g(r) into the approximation for ''c''(''r'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Classical Fluid
Classical fluidsR. Balescu, ''Equilibrium and Nonequilibrium Statistical Mechanics'', (John Wiley, 1975) are systems of particles which retain a definite volume, and are at sufficiently high temperatures (compared to their Fermi energy) that quantum effects can be neglected. A system of hard spheres, interacting only by hard collisions (e.g., billiards, marbles), is a model classical fluid. Such a system is well described by the Percus–Yevik equation. Common liquids, e.g., liquid air, gasoline etc., are essentially mixtures of classical fluids. Electrolytes, molten salts, salts dissolved in water, are classical charged fluids. A classical fluid when cooled undergoes a freezing transition. On heating it undergoes an evaporation transition and becomes a classical gas that obeys Boltzmann statistics. A system of charged classical particles moving in a uniform positive neutralizing background is known as a one-component plasma (OCP). This is well described by the Hyper-netted chain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |