|
Flame Speed
The flame speed is the measured rate of expansion of the flame front in a combustion reaction. Whereas ''flame velocity'' is generally used for a fuel, a related term is explosive velocity, which is the same relationship measured for an explosive. Combustion engineers differentiate between the laminar flame speed and turbulent flame speed. Flame speed is typically measured in m/s, cm/s, etc. In engines In an internal combustion engine, the flame speed of a fuel is a property which determines its ability to undergo controlled combustion without detonation. Flame speed is used along with adiabatic flame temperature to help determine the engine's efficiency. According to one source, "...high flame-speed combustion processes, which closely approximate constant-volume processes, should reflect in high efficiencies.''NASA Technical Note'', May 1977,Emissions and Total Energy Consumption of a Multicylinder Piston Engine Running on Gasoline and a Hydrogen-Gasoline Mixture" The flame spee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame Front
A premixed flame is a flame formed under certain conditions during the combustion of a premixed charge (also called pre-mixture) of fuel and oxidiser. Since the fuel and oxidiser—the key chemical reactants of combustion—are available throughout a homogeneous stoichiometric premixed charge, the combustion process once initiated sustains itself by way of its own heat release. The majority of the chemical transformation in such a combustion process occurs primarily in a thin interfacial region which separates the unburned and the burned gases. The premixed flame interface propagates through the mixture until the entire charge is depleted. The propagation speed of a premixed flame is known as the flame speed (or burning velocity) which depends on the convection-diffusion-reaction balance within the flame, i.e. on its inner chemical structure. The premixed flame is characterised as laminar or turbulent depending on the velocity distribution in the unburned pre-mixture (which provid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detonation
Detonation () is a type of combustion involving a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations propagate supersonically through shock waves with speeds about 1 km/sec and differ from deflagrations which have subsonic flame speeds about 1 m/sec. Detonation may form from an explosion of fuel-oxidizer mixture. Compared with deflagration, detonation doesn't need to have an external oxidizer. Oxidizers and fuel mix when deflagration occurs. Detonation is more destructive than deflagrations. In detonation, the flame front travels through the air-fuel faster than sound; while in deflagration, the flame front travels through the air-fuel slower than sound. Detonations occur in both conventional solid and liquid explosives, as well as in reactive gases. TNT, dynamite, and C4 are examples of high power explosives that detonate. The detonation velocity, velocity of detonation in solid an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wobbe Index
The Wobbe index (WI) or Wobbe number is an indicator of the interchangeability of fuel gases such as natural gas, liquefied petroleum gas (LPG), and town gas and is frequently defined in the specifications of gas supply and transport utilities. If V_C is the higher heating value, or higher calorific value, and G_S is the specific gravity, the Wobbe index, I_W, is defined as: :I_W = \frac . :G_S = \frac = \frac \rho_ is the density of the gas at standard conditions, the definition of which changed in 1982. Published Wobbe data may be using 0 °C, 15 °C, 15.56 °C, 20 °C or 25 °C. EU directives on gas quality use 15 °C in accordance with ISO 13443 and ISO 6976. \rho_ is the density of air at standard conditions, M is the molar mass of the gas and M_ is the molar mass of air which is about 28.96 kg/kmol. . The Wobbe index is used to compare the combustion energy output of different composition fuel gases in an appliance (fire, cooker etc.). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burn Rate (chemistry)
In chemistry, the burn rate (or burning rate) is a measure of the linear combustion rate of a compound or substance such as a candle or a solid propellant. It is measured in length over time, such as millimeters per second or inches per second. Among the variables affecting burn rate are pressure and temperature. Burn rate is an important parameter, especially in propellants, because it determines the rate at which exhaust gases are generated from the burning propellant, which decides the flow rate through the nozzle. The thrust generated in the rocket of a missile depends on this flow rate. Thus, knowing the burn rate of a propellant and how it changes under various conditions is of fundamental importance in the successful design of a solid rocket motor. The concept of burn rate is also relevant in case of liquid propellants. Measurement One device for measuring the burning rate is a V-shaped metal channel about 1–2 feet long wherein a sample is placed, with a cross-sectional d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Equation
G, or g, is the seventh letter of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages, and others worldwide. Its name in English is ''gee'' (pronounced ), plural ''gees''. The lowercase version can be written in two forms: the single-storey (sometimes "opentail") and the double-storey (sometimes "looptail") . The former is commonly used in handwriting and fonts based on it, especially fonts intended to be read by children. History The evolution of the Latin alphabet's G can be traced back to the Latin alphabet's predecessor, the Greek alphabet. The voiced velar stop was represented by the third letter of the Greek alphabet, gamma (Γ), which was later adopted by the Etruscan language. Latin then borrowed this "rounded form" of gamma, C, to represent the same sound in words such as ''recei'', which was likely an early dative form of '' rex'', meaning "king", as found in an "early Latin inscription." Over time, how ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deflagration
Deflagration (Lat: ''de + flagrare'', 'to burn down') is subsonic combustion in which a pre-mixed flame propagates through an explosive or a mixture of fuel and oxidizer. Deflagrations in high and low explosives or fuel–oxidizer mixtures may transition to a detonation depending upon confinement and other factors. Most fires found in daily life are diffusion flames. Deflagrations with flame speeds in the range of 1 m/s differ from detonations which propagate supersonically with detonation velocities in the range of km/s. Applications Deflagrations are often used in engineering applications when the force of the expanding gas is used to move an object such as a projectile down a barrel, or a piston in an internal combustion engine. Deflagration systems and products can also be used in mining, demolition and stone quarrying via gas pressure blasting as a beneficial alternative to high explosives. Terminology of explosive safety When studying or discussing explosive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History The pioneering work of chemical kinetics was done by German chemist Ludwig Wilhelmy in 1850. He experimentally studied the rate of inversion of sucrose and he used integrated rate law for the determination of the reaction kinetics of this reaction. His work was noticed 34 years later by Wilhelm Ostwald. In 1864, Peter Waage and Cato Guldberg published the law ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Flame Temperature
In the study of combustion, the adiabatic flame temperature is the temperature reached by a flame under ideal conditions. It is an upper bound of the temperature that is reached in actual processes. There are two types of Adiabatic process, adiabatic flame temperature: ''constant volume'' and ''constant pressure'', depending on how the process is completed. The constant volume process, constant volume adiabatic flame temperature is the temperature that results from a complete combustion process that occurs without any Work (thermodynamics), work, heat transfer or changes in kinetic energy, kinetic or potential energy. Its temperature is higher than in the ''constant pressure'' process because no energy is utilized to change the volume of the system (i.e., generate work). Common flames In daily life, the vast majority of flames one encounters are those caused by rapid Redox, oxidation of hydrocarbons in materials such as wood, wax, fat, plastics, propane, and gasoline. The consta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
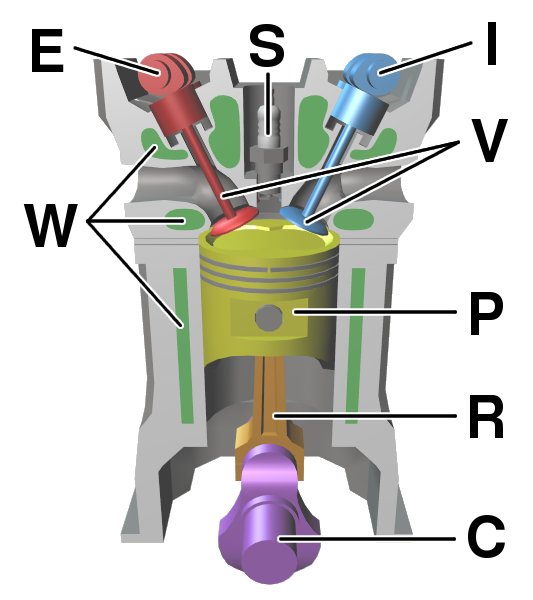
Internal Combustion Engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons (reciprocating engine, piston engine), turbine blades (gas turbine), a Wankel engine, rotor (Wankel engine), or a propulsive nozzle, nozzle (jet engine). This force moves the component over a distance. This process transforms chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to. The first commercially successful internal combustion engines were invented in the mid-19th century. The first modern internal combustion engine, the Otto engine, was designed in 1876 by the German engineer Nicolaus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion (e.g., using a lit match to light a fire), the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science. Combustion is often a complicated sequence of elementary reaction, elementary Radical (chemistry), radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Turbulent
In fluid dynamics, turbulence or turbulent flow is fluid motion characterized by chaotic changes in pressure and flow velocity. It is in contrast to laminar flow, which occurs when a fluid flows in parallel layers with no disruption between those layers. Turbulence is commonly observed in everyday phenomena such as surf, fast flowing rivers, billowing storm clouds, or smoke from a chimney, and most fluid flows occurring in nature or created in engineering applications are turbulent. Turbulence is caused by excessive kinetic energy in parts of a fluid flow, which overcomes the damping effect of the fluid's viscosity. For this reason, turbulence is commonly realized in low viscosity fluids. In general terms, in turbulent flow, unsteady vortices appear of many sizes which interact with each other, consequently drag due to friction effects increases. The onset of turbulence can be predicted by the dimensionless Reynolds number, the ratio of kinetic energy to viscous damping i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laminar Flame Speed
Laminar flame speed is an intrinsic characteristic of premixed combustible mixtures. It is the speed at which an un-stretched laminar flame will propagate through a quiescent mixture of unburned reactants. Laminar flame speed is given the symbol ''s''L. According to the thermal flame theory of Ernest-François Mallard and Le Chatelier, the un-stretched laminar flame speed is dependent on only three properties of a chemical mixture: the thermal diffusivity of the mixture, the reaction rate of the mixture and the temperature through the flame zone: s_\mathrm^ = \sqrt \alpha is thermal diffusivity, \dot is reaction rate, and the temperature subscript u is for unburned, b is for burned and i is for ignition temperature. Laminar flame speed is a property of the mixture (fuel structure, stoichiometry) and thermodynamic conditions upon mixture ignition (pressure, temperature). Turbulent flame speed is a function of the aforementioned parameters, but also heavily depends on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |