|
Cyclobutadiene
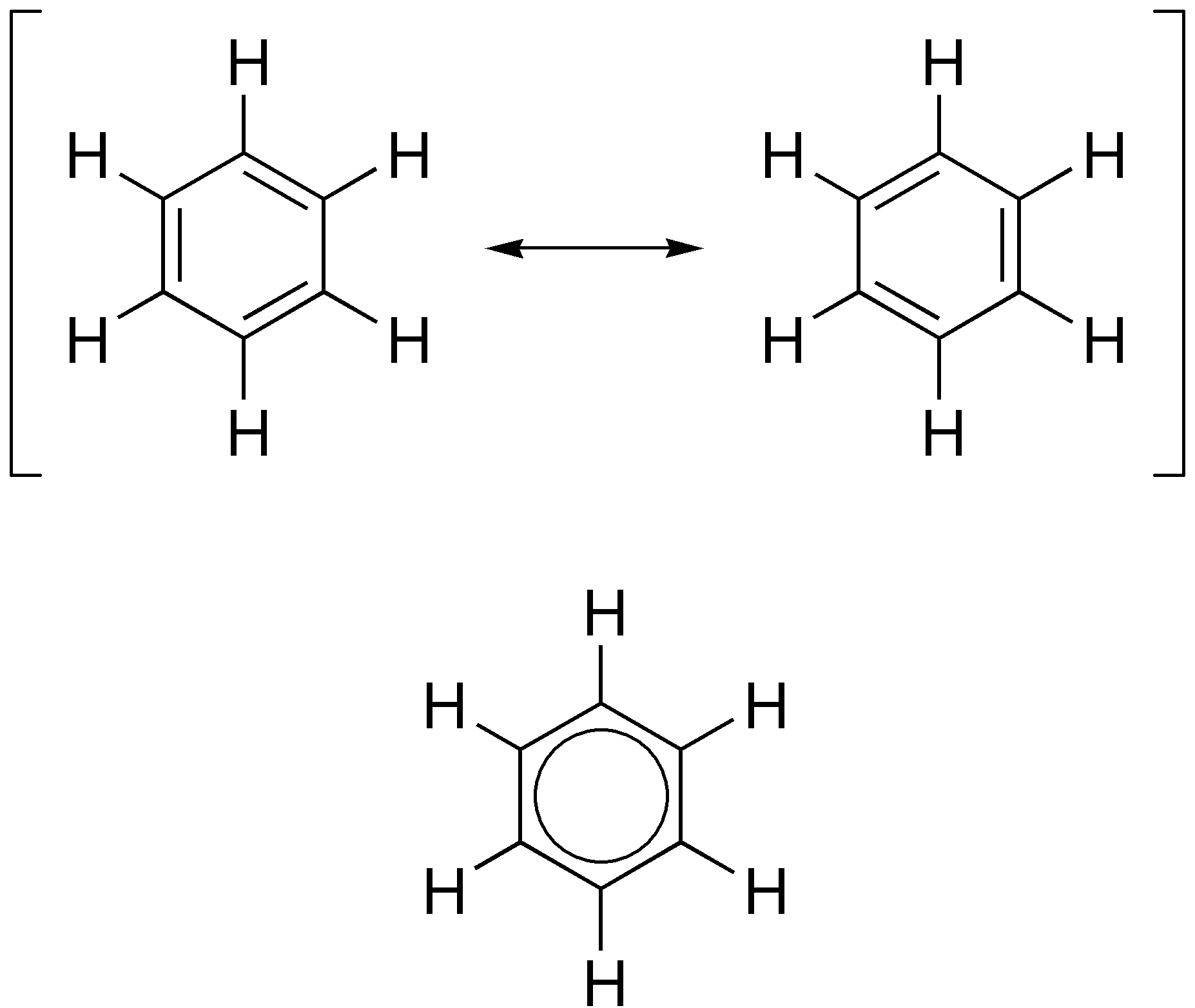
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 pi electrons (or π electrons). It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of a pseudo- (or second order) Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiaromaticity
Antiaromaticity is a chemical property of a cyclic molecule with a pi electron, π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aromatic compounds, which follow Hückel's rule ([4''n''+2] π electrons) and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the Aromatic ring current, diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be #Antiaromaticity in NMR Spectra, observed by NMR spectroscopy. Examples of antiaromatic compounds are pentalene (A), biphenylene (B), cyclopentadienyl cation (C). The prototypical example of antiaromaticity, cyclobutadiene, is the subject of debate, with some scienti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadieneiron Tricarbonyl
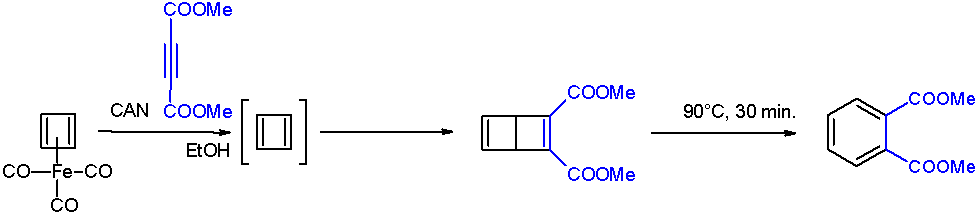
Cyclobutadieneiron tricarbonyl is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow oil that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive species in the free state. Preparation and structure Cyclobutadieneiron tricarbonyl was first prepared in 1965 by Pettit from 3,4-dichlorocyclobutene and diiron nonacarbonyl: :C4H4Cl2 + 2 Fe2(CO)9 → (C4H4)Fe(CO)3 + 2 Fe(CO)5 + 5 CO + FeCl2 The compound is an example of a piano stool complex. The C-C distances are 1.426 Å. Properties Oxidative decomplexation of cyclobutadiene is achieved by treating the tricarbonyl complex with ceric ammonium nitrate. The released cyclobutadiene is trapped with a quinone, which functions as a dienophile. Cyclobutadieneiron tricarbonyl displays aromaticity as evidenced by some of its reactions, which can be classified as electrophilic aromatic substitution: : It undergoes Friedel-Crafts acylation with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedrane
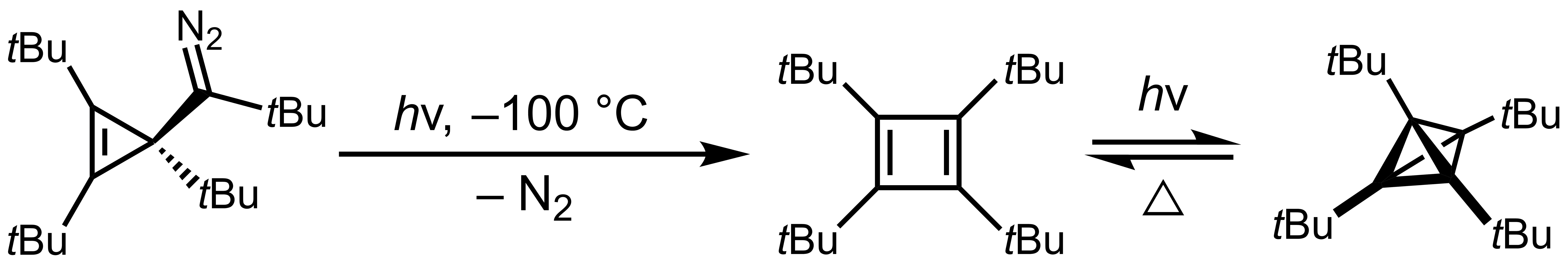
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized . However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. C4 tetrahedranes Tetrahedrane () is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane remains elusive, although predicted kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene with atomic carbon. Contrariwise, several organic compounds with the tetrahedrane core are known. All have multiply bulky substituents, ''tert''-butyl (''t''-Bu) or larger. Locking a tetrahedrane molecule inside a fullerene has only been attempted ''in silico''. All known syntheses have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trapping Reagent
In chemistry, a chemical trap is a chemical compound that is used to detect unstable compounds. The method relies on efficiency of bimolecular reactions with reagents to produce a more easily characterize trapped product. In some cases, the trapping agent is used in large excess. Case studies Cyclobutadiene A famous example is the detection of cyclobutadiene released upon oxidation of cyclobutadieneiron tricarbonyl. When this degradation is conducted in the presence of an alkyne, the cyclobutadiene is trapped as a bicyclohexadiene. The requirement for this trapping experiment is that the oxidant (ceric ammonium nitrate) and the trapping agent be mutually compatible. : Diphosphorus Diphosphorus is an old target of chemists since it is the heavy analogue of N2. Its fleeting existence is inferred by the controlled degradation of certain niobium complexes in the presence of trapping agents. Again, a Diels-Alder strategy is employed in the trapping: : Silylene Another classic but el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromaticity
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annulene
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds (' mancude'). They have the general formula C''n''H''n'' (when ''n'' is an even number) or C''n''H''n''+1 (when ''n'' is an odd number). The IUPAC accepts the use of 'annulene nomenclature' in naming carbocyclic ring systems with 7 or more carbon atoms, using the name ' 'n''nnulene' for the mancude hydrocarbon with ''n'' carbon atoms in its ring, though in certain contexts (e.g., discussions of aromaticity for different ring sizes), smaller rings (''n'' = 3 to 6) can also be informally referred to as annulenes. Using this form of nomenclature 1,3,5,7-cyclooctatetraene is nnulene and benzene is nnulene (and occasionally referred to as just 'annulene'). The discovery that 8nnulene possesses a number of key properties associated with other aromatic molecules was an important development in the understanding of aromaticity as a chemical concept. In the related annul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jahn–Teller Effect
The Jahn–Teller effect (JT effect or JTE) is an important mechanism of spontaneous symmetry breaking in molecular and solid-state systems which has far-reaching consequences in different fields, and is responsible for a variety of phenomena in spectroscopy, stereochemistry, crystal chemistry, molecular and solid-state physics, and materials science. The effect is named for Hermann Arthur Jahn and Edward Teller, who first reported studies about it in 1937. Simplified overview The Jahn–Teller effect, sometimes also referred to as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that results from certain electron configurations. The Jahn–Teller theorem essentially states that any non-linear molecule with a spatially degenerate energy level, degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the species. For a description of another type of geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carceplex
In host–guest chemistry, a carceplex is a class of chemical structures in the carcerand family that are hinged, and can be closed using reagents that react with the carceplex and trap precursors of reactive intermediates, and are unreactive with the trapped precursor or reactive intermediate. This is useful for determining the spectroscopic and crystallographic properties of reactive intermediates in relative isolation, particularly compounds prone to dimerization like cyclobutadiene Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is .... References {{Reflist, refs= {{cite book, last=Cram, first=Donald, title=Container molecules and their guests, year=1997, publisher=Royal Society of Chemistry, location=Cambridge, UK, isbn=0-85404-507-4, url=https://books.google.com/books?id=RqE5G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Isomer
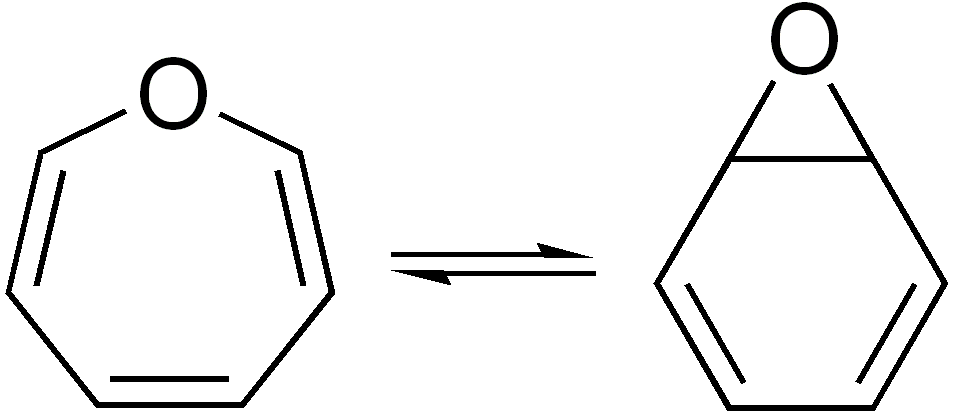
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions. Benzene There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally proposed for benzene itself before the actual structure of benzene was known. Others were later synthesized in lab. Some have been observed to isomerize to benzene, whereas others tend to undergo other reactions instead, or isomerize by ways other than pericyclic reactions. Image:Benzene-2D-flat.png, Benzene Image:Historic Benzene Formulae Dewar(1867) V.1.svg, Dewar benzene Image:Prisman2.svg, Prismane Image:Benzvalene.png, Benzvalene Image:Bicycloprop-2-enyl.svg, Bicyclopropenyl Cyclooctatetraene The valence isomers are not restricted to isomers of benzene. Valence isomers are also seen in the series (CH)8. Due to the larger number of units, the number of possible valence isomers is also greater and at least 21: Image:Cyclooc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hückel's Rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4''n'' + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time. In agreement with the Möbius–Hückel concept, a cyclic ring molecule follows Hückel's rule when the number of its π-electrons equals 4''n'' + 2, although clearcut examples are really only established for values of ''n'' = 0 up to about ''n'' = 6. Hückel's rule was originally based on calculations using the Hückel method, although it can also be justified by considering a particle in a ring system, by the LCAO method and by the Pariser–Parr–Pople method. Aroma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effect
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |