|
Carceplex
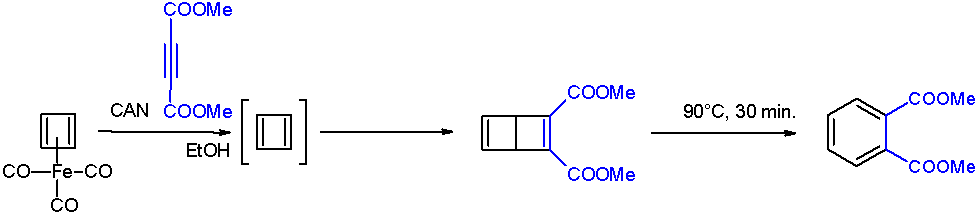
In host–guest chemistry, a carceplex is a class of chemical structures in the carcerand family that are hinged, and can be closed using reagents that react with the carceplex and trap precursors of reactive intermediates, and are unreactive with the trapped precursor or reactive intermediate. This is useful for determining the spectroscopic and crystallographic properties of reactive intermediates in relative isolation, particularly compounds prone to dimerization like cyclobutadiene Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is .... References {{Reflist, refs= {{cite book, last=Cram, first=Donald, title=Container molecules and their guests, year=1997, publisher=Royal Society of Chemistry, location=Cambridge, UK, isbn=0-85404-507-4, url=https://books.google.com/books?id=RqE ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Host–guest Chemistry
In supramolecular chemistry, host–guest chemistry describes inclusion compound, complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. Although non-covalent interactions could be roughly divided into those with more electrostatic or dispersive contributions, there are few commonly mentioned types of non-covalent interactions: ionic bonding, hydrogen bond, hydrogen bonding, van der Waals forces and hydrophobic effect, hydrophobic interactions. Overview Host–guest chemistry is a branch of supramolecular chemistry in which a host mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcerand
In host–guest chemistry, a carcerand () is a host molecule that completely entraps its guest (which can be an ion, atom or other chemical species) so that it will not escape even at high temperatures. This type of molecule was first described in 1985 by Donald J. Cram and coworkers. The complexes formed by a carcerand with permanently imprisoned guests are called carceplexes. In contrast, hemicarcerands allow guests to enter and exit the cavity at high temperatures but will form stable complexes at ambient temperatures. The complexes formed by a hemicarcerand and a guest are called ''hemicarceplexes''. Reactivity of bound guests Cram described the interior of the container compound as the inner phase in which radically different reactivity was observed. He used a hemicarcerand to isolate highly unstable, antiaromatic cylobutadiene at room temperature. The hemicarcerand stabilizes guests within its cavity by preventing their reaction with other molecules... Synthesis The fir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hinge
A hinge is a mechanical bearing that connects two solid objects, typically allowing only a limited angle of rotation between them. Two objects connected by an ideal hinge rotate relative to each other about a fixed axis of rotation: all other translations or rotations being prevented, and thus a hinge has one degree of freedom. Hinges may be made of flexible material or of moving components. In biology, many joints function as hinges, like the elbow joint. History Ancient remains of stone, marble, wood, and bronze hinges have been found. Some date back to at least Ancient Egypt. In Ancient Rome, hinges were called cardō and gave name to the goddess Cardea and the main street Cardo. This name cardō lives on figuratively today as "the chief thing (on which something turns or depends)" in words such as ''cardinal''. According to the OED, the English word hinge is related to '' hang''. Door hinges ; Barrel hinge: A barrel hinge consists of a sectional barrel (the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place. Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopic
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallographic
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The word "crystallography" is derived from the Greek word κρύσταλλος (''krystallos'') "clear ice, rock-crystal", with its meaning extending to all solids with some degree of transparency, and γράφειν (''graphein'') "to write". In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming that 2014 would be the International Year of Crystallography. denote a direction vector (in real space). * Coordinates in ''angle brackets'' or ''chevrons'' such as <100> denote a ''family'' of directions which are related by symmetry operations. In the cubic crystal system for example, would mean 00 10 01/nowiki> or the negative of any of those directions. * Miller indices in ''parentheses' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimerization
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n'' annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)