|
Cinnamaldehyde
Cinnamaldehyde is an organic compound with the formula or . Occurring naturally as predominantly the ''trans'' (''E'') isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus '' Cinnamomum''. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is '' trans''-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an α,β-unsaturated carbonyl compound. Its color is due to the π → π* transition: increased conjugation in comparison with acrolein shifts this band towa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
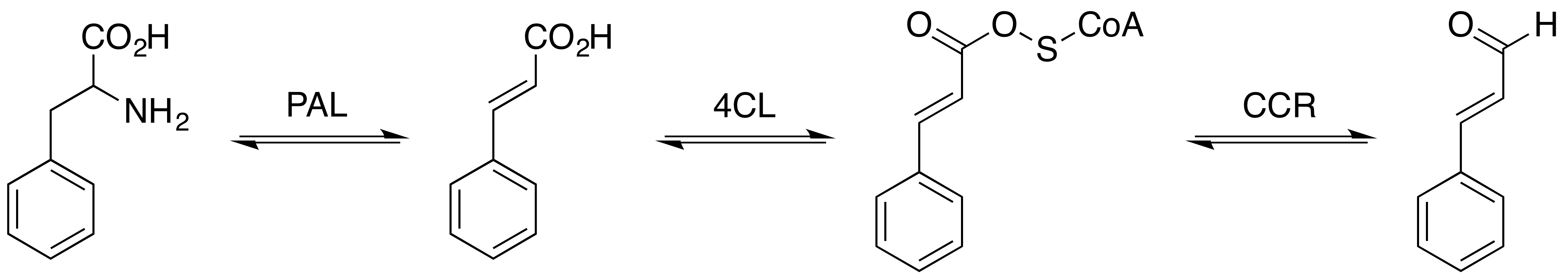
Cinnamaldehyde Biosynthesis Pathway
Cinnamaldehyde is an organic compound with the formula or . Occurring naturally as predominantly the Cis-trans isomerism, ''trans'' (''E'') isomer, it gives cinnamon its Flavoring, flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, Viscosity, viscous liquid occurs in the Bark (botany), bark of cinnamon trees and other species of the genus ''Cinnamomum''. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''Geometric isomerism, trans''-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an α,β-Unsaturated carbonyl compound, α,β-unsaturated carbonyl compound. Its color is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful. It is a colorless liquid with a characteristic almond-like odor, and is commonly used in cherry-flavored sodas. A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods. History Benzaldehyde was first extracted in 1803 by the French pharmacist Martrès. His experiments focused on elucidating the nature of amygdalin, the poisonous compound found in bitter almonds, the fruit of '' Prunus dulcis''. Further work on the oil by Pierre Robiquet and Antoine Boutron Charlard, two French chemists, produced benzaldehyde. In 1832, Friedrich Wöhler and Justus von Liebig first synthesized benzaldehyde. Production Benzaldeh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cinnamic Acid
Cinnamic acid is an organic compound with the formula phenyl, C6H5-CH=CH-Carboxylic acid, COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a Cis–trans isomerism, ''cis'' and a ''trans'' isomer, although the latter is more common. The ''cis''-isomer is called allocinnamic acid. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cinnamon
Cinnamon is a spice obtained from the inner bark of several tree species from the genus ''Cinnamomum''. Cinnamon is used mainly as an aromatic condiment and flavouring additive in a wide variety of cuisines, sweet and savoury dishes, biscuits, breakfast cereals, Snack, snack foods, bagels, teas, hot chocolate and traditional foods. The aroma and flavour of cinnamon derive from its essential oil and principal component, cinnamaldehyde, as well as numerous other constituents, including eugenol. Cinnamon is the name for several species of trees and the commercial spice products that some of them produce. All are members of the genus ''Cinnamomum'' in the family Lauraceae. Only a few ''Cinnamomum'' species are grown commercially for spice. ''Cinnamomum verum'' (alternatively ''C. zeylanicum''), known as "Ceylon cinnamon" after its origins in Sri Lanka (formerly Ceylon), is considered to be "true cinnamon", but most cinnamon in international commerce is derived from four other speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cinnamyl Alcohol
Cinnamyl alcohol or styron is an organic compound that is found in esterified form in storax, Balsam of Peru, and cinnamon leaves. It forms a white crystalline solid when pure, or a yellow oil when even slightly impure. It can be produced by the hydrolysis of storax. Cinnamyl alcohol occurs naturally only in small quantities, so its industrial demand is usually fulfilled by chemical synthesis starting from cinnamaldehyde. Properties The compound is a solid at room temperature, forming colorless crystals that melt upon gentle heating. As is typical of most higher-molecular weight alcohols, it is sparingly soluble in water at room temperature, but highly soluble in most common organic solvents. Uses Cinnamyl alcohol has a distinctive odor described as "sweet, balsam, hyacinth, spicy, green, powdery, cinnamic" and is used in perfumery and as a deodorant. Cinnamyl alcohol is the starting material used in the synthesis of reboxetine. Safety Cinnamyl alcohol has been found to have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
α,β-Unsaturated Carbonyl Compound
α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ−R. Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds, the carbonyl group is conjugated with an alkene (hence the adjective unsaturated). Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction. Classifications α,β-Unsaturated carbonyl compounds can be subclassified according to the nature of the carbonyl and alkene groups. File:Methyl vinyl ketone.svg, Methyl vinyl ketone, the simplest α,β-unsaturated ketone File:Acrolein-2D-skeletal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phenylpropanoid
The phenylpropanoids are a diverse family of organic compounds that are biosynthesized by plants from the amino acids phenylalanine and tyrosine in the shikimic acid pathway. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-Coumaroyl-CoA, 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including Monolignol, lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and also mediate plant-pollinator interactions as floral pigments and scent compounds. Hydroxycinnamic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Essential Oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does ''not'' mean required or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism. Essential oils are generally extracted by distillation, often by using steam. Other processes include expression, solvent extraction, '' sfumatura'', absolute oil extraction, resin tapping, wax embedding, and cold pressing. They are used in perfumes, cosmetics, soaps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol (drug), alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. International Agency for Research on Cancer, The International Agency for Research on Cancer (IARC) has listed acetaldehyde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Odor
An odor (American English) or odour ( Commonwealth English; see spelling differences) is a smell or a scent caused by one or more volatilized chemical compounds generally found in low concentrations that humans and many animals can perceive via their olfactory system. While ''smell'' can refer to pleasant and unpleasant odors, the terms ''scent'', ''aroma'', and ''fragrance'' are usually reserved for pleasant-smelling odors and are frequently used in the food and cosmetic industry to describe floral scents or to refer to perfumes. Odor physiology Sense of smell The perception of odors, or sense of smell, is mediated by the olfactory nerve. The olfactory receptor (OR) cells are neurons present in the olfactory epithelium, which is a small patch of tissue at the back of the nasal cavity. There are millions of olfactory receptor neurons that act as sensory signaling cells. Each neuron has cilia in direct contact with the air. Odorous molecules bind to receptor pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |