|
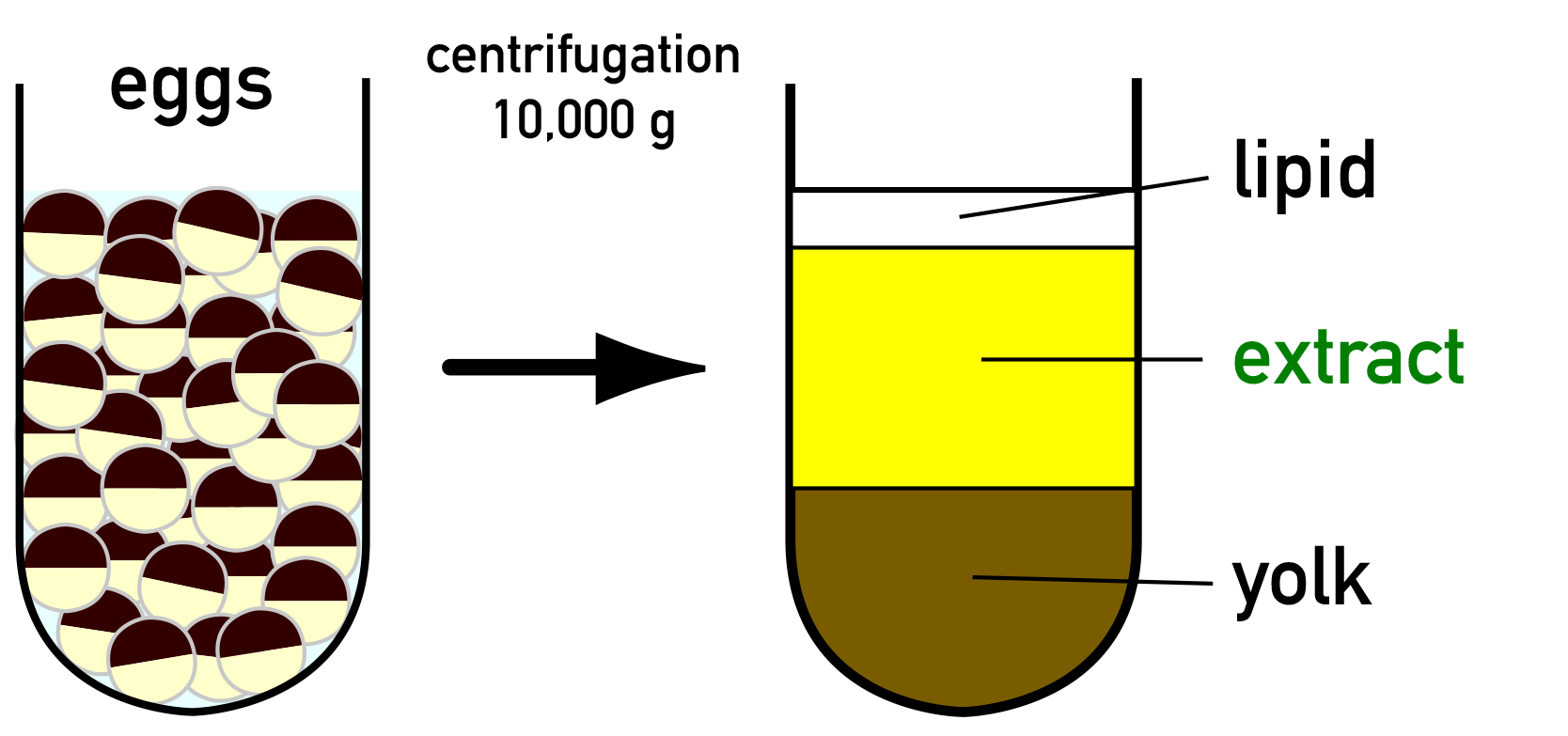
Xenopus Egg Extract
''Xenopus'' egg extract is a lysate that is prepared by crushing the eggs of the African clawed frog ''Xenopus laevis''. It offers a powerful cell-free (or ''in vitro'') system for studying various cell biological processes, including cell cycle progression, nuclear transport, DNA replication and chromosome segregation. It is also called ''Xenopus'' egg cell-free system or ''Xenopus'' egg cell-free extract. History The first frog egg extract was reported in 1983 by Lohka and Masui. This pioneering work used eggs of the Northern leopard frog ''Rana pipiens'' to prepare an extract. Later, the same procedure was applied to eggs of ''Xenopus laevis'', becoming popular for studying cell cycle progression and cell cycle-dependent cellular events. Extracts derived from eggs of the Japanese common toad ''Bufo japonicus'' or of the Western clawed frog ''Xenopus tropicalis'' have also been reported. Basics of extract preparation The cell cycle of unfertilized eggs of ''X. laevis'' is arrested ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
African Clawed Frog
The African clawed frog (''Xenopus laevis'', also known as the xenopus, African clawed toad, African claw-toed frog or the ''platanna'') is a species of African aquatic frog of the family Pipidae. Its name is derived from the three short claws on each hind foot, which it uses to tear apart its food. The word ''Xenopus'' means 'strange foot' and ''laevis'' means 'smooth'. The species is found throughout much of Sub-Saharan Africa (Nigeria and Sudan to South Africa),Weldon; du Preez; Hyatt; Muller; and Speare (2004). Origin of the Amphibian Chytrid Fungus.' Emerging Infectious Diseases 10(12). and in isolated, introduced populations in North America, South America, Europe, and Asia. All species of the family Pipidae are tongueless, toothless and completely aquatic. They use their hands to shove food in their mouths and down their throats and a hyobranchial pump to draw or suck things in their mouth. Pipidae have powerful legs for swimming and lunging after food. They also use the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelator
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yoshio Masui
is a Japanese Canadian cell biologist. Masui retired in 1997 and has since held the position of Professor Emeritus at the University of Toronto. Education Masui studied biology at Kyoto University, graduating with his Bachelor of Science degree in zoology in 1953, his Master of Science in 1955 and his Ph.D. in 1961. Career and research While still studying at Kyoto University, he taught biology, first as a teacher's assistant and then as a teacher, at Konan University, where he was promoted to Assistant Professor after his earning his Ph.D. In 1966, he moved to Yale University to join Clement L. Markert's lab, and in 1969 to the University of Toronto, where he taught as Associate Professor in the Department of Zoology. Recognition In 1998, he won the Albert Lasker Award for Basic Medical Research with Lee Hartwell and Paul Nurse for their pioneering work on cell division. He was elected a List of Fellows of the Royal Society elected in 1998, Fellow of the Royal Society (FRS) in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Establishment Of Sister Chromatid Cohesion
Sister chromatid cohesion refers to the process by which sister chromatids are paired and held together during certain phases of the cell cycle. Establishment of sister chromatid cohesion is the process by which chromatin-associated cohesin protein becomes competent to physically bind together the sister chromatids. In general, cohesion is established during S phase as DNA is replicated, and is lost when chromosomes segregate during mitosis and meiosis. Some studies have suggested that cohesion aids in aligning the kinetochores during mitosis by forcing the kinetochores to face opposite cell poles. Cohesin loading Cohesin first associates with the chromosomes during G1 phase. The cohesin ring is composed of two SMC (structural maintenance of chromosomes) proteins and two additional Scc proteins. Cohesin may originally interact with chromosomes via the ATPase domains of the SMC proteins. In yeast, the loading of cohesin on the chromosomes depends on proteins Scc2 and Scc4. Cohesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cohesin
Cohesin is a protein complex that mediates sister chromatid cohesion, homologous recombination, and DNA looping. Cohesin is formed of SMC3, SMC1, SCC1 and SCC3 ( SA1 or SA2 in humans). Cohesin holds sister chromatids together after DNA replication until anaphase when removal of cohesin leads to separation of sister chromatids. The complex forms a ring-like structure and it is believed that sister chromatids are held together by entrapment inside the cohesin ring. Cohesin is a member of the SMC family of protein complexes which includes Condensin, MukBEF and SMC-ScpAB. Cohesin was separately discovered in budding yeast by Douglas Koshland and Kim Nasmyth. Structure Cohesin is a multi-subunit protein complex, made up of SMC1, SMC3, RAD21 and SCC3 (SA1 or SA2). SMC1 and SMC3 are members of the Structural Maintenance of Chromosomes (SMC) family. SMC proteins have two main structural characteristics: an ATP-binding cassette-like 'head' domain with ATPase activity ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensin
Condensins are large protein complexes that play a central role in chromosome assembly and segregation during mitosis and meiosis (Figure 1). Their subunits were originally identified as major components of mitotic chromosomes assembled in ''Xenopus'' egg extracts. Subunit composition Eukaryotic types Many eukaryotic cells possess two different types of condensin complexes, known as condensin I and condensin II, each of which is composed of five subunits (Figure 2). Condensins I and II share the same pair of core subunits, SMC2 and SMC4, both belonging to a large family of chromosomal ATPases, known as SMC proteins (SMC stands for Structural Maintenance of Chromosomes). Each of the complexes contains a distinct set of non-SMC regulatory subunits (a kleisin subunit and a pair of HEAT repeat subunits). Both complexes are large, having a total molecular mass of 650-700 kDa. The core subunits condensins (SMC2 and SMC4) are conserved among all eukaryotic species that have bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
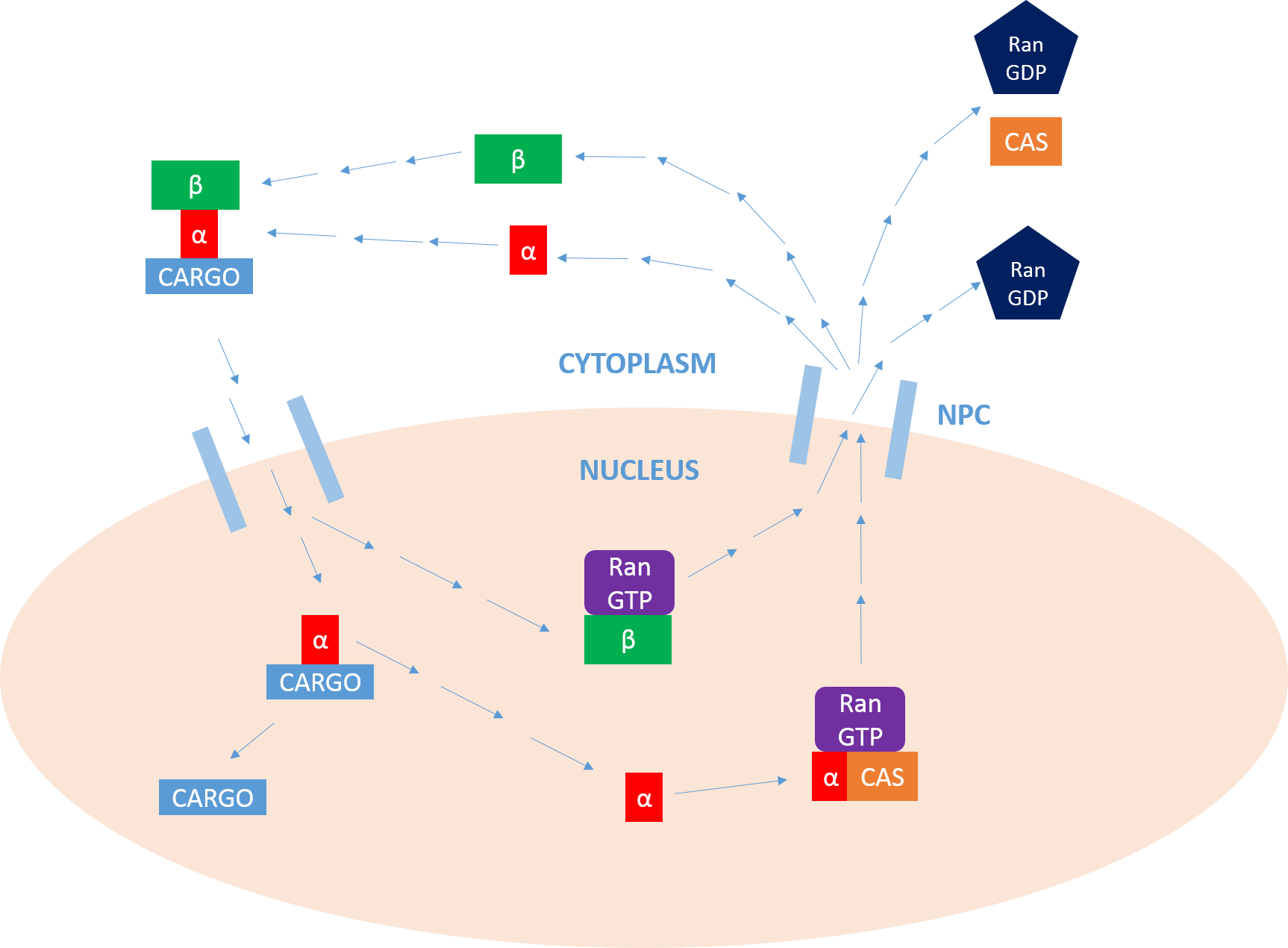
Importin
Importin is a type of karyopherin that transports protein molecules from the cell's cytoplasm to the nucleus. It does so by binding to specific recognition sequences, called nuclear localization sequences (NLS). Importin has two subunits, importin α and importin β. Members of the importin-β family can bind and transport cargo by themselves, or can form heterodimers with importin-α. As part of a heterodimer, importin-β mediates interactions with the pore complex, while importin-α acts as an adaptor protein to bind the nuclear localization signal (NLS) on the cargo. The NLS-Importin α-Importin β trimer dissociates after binding to Ran GTP inside the nucleus, with the two importin proteins being recycled to the cytoplasm for further use. Discovery Importin can exist as either a heterodimer of importin-α/β or as a monomer of Importin-β. Importin-α was first isolated in 1994 by a group includinEnno Hartmann based at the Max Delbrück Center for Molecular Medicine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Licensing Factor
A licensing factor is a protein or complex of proteins that allows an origin of replication to begin DNA replication at that site. Licensing factors primarily occur in eukaryotic cells, since bacteria use simpler systems to initiate replication. However, many archaea use homologues of eukaryotic licensing factors to initiate replication. Function Origins of replication represent start sites for DNA replication and so their "firing" must be regulated to maintain the correct karyotype of the cell in question. The origins are required to fire only once per cell cycle, an observation that led to the postulated existence of licensing factors by biologists in the first place. If the origins were not carefully regulated then DNA replication could be restarted at that origin giving rise to multiple copies of a section of DNA. This could be damaging to cells and could have detrimental effects on the organism as a whole. The control that licensing factors exert over the cycle represents a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
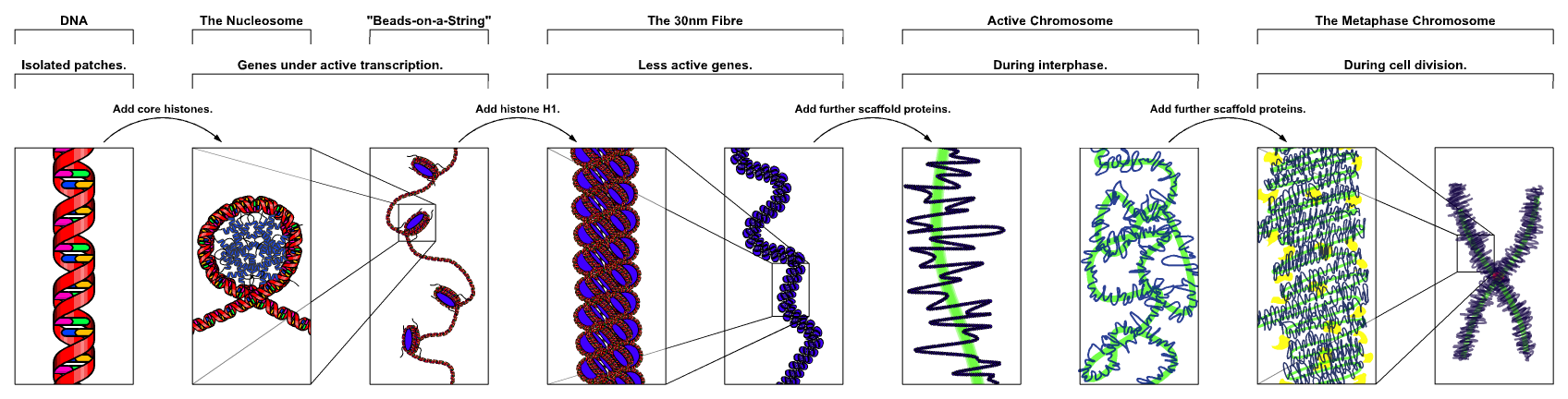
Chromatin
Chromatin is a complex of DNA and protein found in eukaryote, eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in reinforcing the DNA during cell division, preventing DNA repair#DNA damage, DNA damage, and regulating gene expression and DNA replication. During mitosis and meiosis, chromatin facilitates proper segregation of the chromosomes in anaphase; the characteristic shapes of chromosomes visible during this stage are the result of DNA being coiled into highly condensed chromatin. The primary protein components of chromatin are histones. An octamer of two sets of four histone cores (Histone H2A, Histone H2B, Histone H3, and Histone H4) bind to DNA and function as "anchors" around which the strands are wound.Maeshima, K., Ide, S., & Babokhov, M. (2019). Dynamic chromatin organization without the 30-nm fiber. ''Current opinion in cell biolo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spindle Apparatus
In cell biology, the spindle apparatus refers to the cytoskeletal structure of eukaryotic cells that forms during cell division to separate sister chromatids between daughter cells. It is referred to as the mitotic spindle during mitosis, a process that produces genetically identical daughter cells, or the meiotic spindle during meiosis, a process that produces gametes with half the number of chromosomes of the parent cell. Besides chromosomes, the spindle apparatus is composed of hundreds of proteins. Microtubules comprise the most abundant components of the machinery. Spindle structure Attachment of microtubules to chromosomes is mediated by kinetochores, which actively monitor spindle formation and prevent premature anaphase onset. Microtubule polymerization and depolymerization dynamic drive chromosome congression. Depolymerization of microtubules generates tension at kinetochores; bipolar attachment of sister kinetochores to microtubules emanating from opposite ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cdk1
Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine protein kinase, and is a key player in cell cycle regulation. It has been highly studied in the budding yeast '' S. cerevisiae'', and the fission yeast '' S. pombe'', where it is encoded by genes ''cdc28'' an''cdc2'' respectively. With its cyclin partners, Cdk1 forms complexes that phosphorylate a variety of target substrates (over 75 have been identified in budding yeast); phosphorylation of these proteins leads to cell cycle progression. Structure Cdk1 is a small protein (approximately 34 kilodaltons), and is highly conserved. The human homolog of Cdk1, ''CDK1'', shares approximately 63% amino-acid identity with its yeast homolog. Furthermore, human ''CDK1'' is capable of rescuing fission yeast carrying a ''cdc2'' mutation. Cdk1 is comprised mostly by the bare protein kinase motif, which other protein kinases share. Cdk1, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |