|
Volta Potential
The Volta potential (also called Volta effect, Volta potential difference, contact potential difference, outer potential difference, Δψ, or "delta psi") in electrochemistry, is the electrostatic potential difference between two metals (or one metal and one electrolyte) that are in contact and are in thermodynamic equilibrium. Specifically, it is the potential difference between a point close to the surface of the first metal and a point close to the surface of the second metal (or electrolyte). The Volta potential is named after Alessandro Volta. Description When two metals are electrically isolated from each other, an arbitrary potential difference may exist between them. However, when two different neutral metal surfaces are brought into electrical contact (even indirectly, say, through a long electro-conductive wire), electrons will flow from the metal with the higher Fermi level to the metal with the lower Fermi level until the Fermi levels in the two phases are equal. On ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in Electroless nickel-phosphorus plating, electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic chemical species, species in a Solution (chemistry), solution). When a chemical reaction is driven by an electrical Voltage, potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Force Microscopy
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit. Overview Atomic force microscopy (AFM) gathers information by "feeling" or "touching" the surface with a mechanical probe. Piezoelectric elements that facilitate tiny but accurate and precise movements on (electronic) command enable precise scanning. Despite the name, the Atomic Force Microscope does not use the nuclear force. Abilities and spatial resolution The AFM has three major abilities: force measurement, topographic imaging, and manipulation. In force measurement, AFMs can be used to measure the forces between the probe and the sample as a function of their mutual separation. This can be applied to perform force spectroscopy, to measure the mechanical properties of the sample, such as the sample's Youn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Concepts
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in Electroless nickel-phosphorus plating, electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic chemical species, species in a Solution (chemistry), solution). When a chemical reaction is driven by an electrical Voltage, potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volta Effect
The Volta potential (also called Volta effect, Volta potential difference, contact potential difference, outer potential difference, Δψ, or "delta psi") in electrochemistry, is the electrostatic potential difference between two metals (or one metal and one electrolyte) that are in contact and are in thermodynamic equilibrium. Specifically, it is the potential difference between a point close to the surface of the first metal and a point close to the surface of the second metal (or electrolyte). The Volta potential is named after Alessandro Volta. Description When two metals are electrically isolated from each other, an arbitrary potential difference may exist between them. However, when two different neutral metal surfaces are brought into electrical contact (even indirectly, say, through a long electro-conductive wire), electrons will flow from the metal with the higher Fermi level to the metal with the lower Fermi level until the Fermi levels in the two phases are equal. On ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volt
The volt (symbol: V) is the unit of electric potential, Voltage#Galvani potential vs. electrochemical potential, electric potential difference (voltage), and electromotive force in the International System of Units, International System of Units (SI). Definition One volt is defined as the electric potential between two points of a electrical conductor, conducting wire when an electric current of one ampere dissipates one watt of power (physics), power between those points. It can be expressed in terms of SI base units (metre, m, kilogram, kg, second, s, and ampere, A) as : \text = \frac = \frac = \frac = \text\text^2\text^. Equivalently, it is the potential difference between two points that will impart one joule of energy per coulomb of charge that passes through it. It can be expressed in terms of SI base units (metre, m, kilogram, kg, second, s, and ampere, A) as : \text = \frac = \frac = \frac = \text\text^2\text^. It can also be expressed as amperes times ohms (curre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
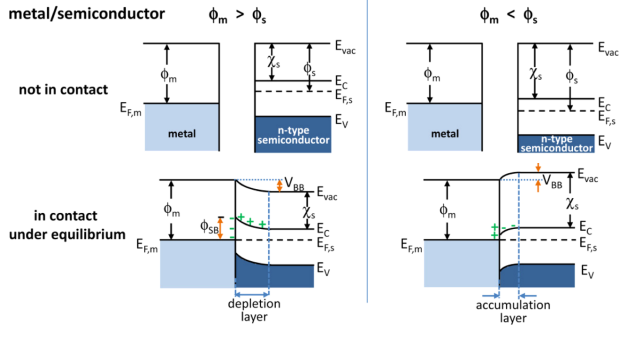
Band Bending
In solid-state physics, band bending refers to the process in which the electronic band structure in a material curves up or down near a junction or interface. It does not involve any physical (spatial) bending. When the electrochemical potential of the free charge carriers around an interface of a semiconductor is dissimilar, charge carriers are transferred between the two materials until an equilibrium state is reached whereby the potential difference vanishes. The band bending concept was first developed in 1938 when Nevill Francis Mott, Mott, Alexander Davydov (physicist), Davydov and Walter H. Schottky, Schottky all published theories of the rectifying effect of Metal–semiconductor junction, metal-semiconductor contacts. The use of semiconductor junctions sparked the computer revolution in the second half of the 20th century. Devices such as the diode, the transistor, the Photoelectric sensor, photocell and many more play crucial roles in technology. Qualitative description ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potential Difference
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to move a positive test charge from the first point to the second point. In the International System of Units (SI), the derived unit for voltage is the ''volt'' (''V''). The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in a generator). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. Since it is the difference in electric potential, it is a physical scalar quantity. A voltmeter can be used to measure the voltage between two points in a system. Often a common reference potential su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvani Potential
In electrochemistry, the Galvani potential (also called Galvani potential difference, or inner potential difference, Δφ, delta phi) is the electric potential difference between two points in the bulk of two phases. These phases can be two different solids (e.g., two metals joined), or a solid and a liquid (e.g., a metal electrode submerged in an electrolyte). The Galvani potential is named after Luigi Galvani. Galvani potential between two metals First, consider the Galvani potential between two metals. When two metals are electrically isolated from each other, an arbitrary voltage difference may exist between them. However, when two different metals are brought into electronic contact, electrons will flow from the metal with a lower voltage to the metal with the higher voltage until the Fermi level of the electrons in the bulk of both phases are equal. The actual numbers of electrons that passes between the two phases is small (it depends on the capacitance between the objects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Potential
Electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as electric potential energy per unit of electric charge. More precisely, electric potential is the amount of work (physics), work needed to move a test charge from a reference point to a specific point in a static electric field. The test charge used is small enough that disturbance to the field is unnoticeable, and its motion across the field is supposed to proceed with negligible acceleration, so as to avoid the test charge acquiring kinetic energy or producing radiation. By definition, the electric potential at the reference point is zero units. Typically, the reference point is Earth (electricity), earth or a point at infinity, although any point can be used. In classical electrostatics, the electrostatic field is a vector quantity expressed as the gradient of the electrostatic potential, which is a scalar (physics), scalar quantity denoted by or occasi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absolute Electrode Potential
Absolute electrode potential, in electrochemistry, according to an IUPAC definition, is the electrode potential of a metal measured with respect to a universal reference system (without any additional metal–solution interface). Definition According to a more specific definition presented by Trasatti, the absolute electrode potential is the difference in electronic energy between a point inside the metal (Fermi level) of an electrode and a point outside the electrolyte in which the electrode is submerged (an electron at rest in vacuum just above the electrolyte surface). This potential is difficult to determine accurately. For this reason, a standard hydrogen electrode is typically used for reference potential. The absolute potential of the SHE is 4.44 ± 0.02 V at 25 °C. Therefore, for any electrode at 25 °C: :E^M_ = E^M_+(4.44 \pm 0.02)\ where: : is electrode potential :V is the unit volt :''M'' denotes the electrode made of metal M :(abs) denot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode Potential
An electrode is an electrical conductor used to make contact with a nonmetallic part of a Electronic circuit, circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The Electrophorus, electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any Electric battery, battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scanning Kelvin Probe
Kelvin probe force microscopy (KPFM), also known as surface potential microscopy, is a noncontact variant of atomic force microscopy (AFM). By raster scanning in the x,y plane the work function of the sample can be locally mapped for correlation with sample features. When there is little or no magnification, this approach can be described as using a scanning Kelvin probe (SKP). These techniques are predominantly used to measure corrosion and coatings. With KPFM, the work function of surfaces can be observed at atomic or molecular scales. The work function relates to many surface phenomena, including catalytic activity, reconstruction of surfaces, doping and band-bending of semiconductors, charge trapping in dielectrics and corrosion. The map of the work function produced by KPFM gives information about the composition and electronic state of the local structures on the surface of a solid. History The SKP technique is based on parallel plate capacitor experiments performed b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |