|
Vilsmaier Reagent
The Vilsmeier reagent is an organic compound with the formula CH3)2NCHCll. It is a salt consisting of the N,N-dimethyliminium cation ( CH3)2N=CHClsup>+) and chloride anion. Depending on the particular reaction, the anion can vary. In typical POCl3-based reactions, the anion is PO2Cl2−. The iminium cation CH3)2N=CHClsup>+ is the reactive component of interest. This iminium species is a derivative of the imidoyl chloride CH3N=CHCl. Analogues of this particular reagent are generated when tertiary amides other than DMF are treated with POCl3. The salt is a white solid that is soluble in polar organic solvents. Vilsmeier reagent is the active intermediate in the formylation reactions, the Vilsmeier reaction or Vilsmeier-Haack reaction that use mixtures of dimethylformamide and phosphorus oxychloride Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminium Cation
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology. Structure Iminium cations adopt alkene-like geometries: the central C=N unit is nearly coplanar with all four substituents. Unsymmetrical iminium cations can exist as cis and trans isomers. The C=N bonds, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed. The C=N distance is slightly shorter in iminium cations than in the parent imine, and computational studies indicate that the C=N bonding is also stronger in iminium vs imine, although the C=N distance contracts only slightly. These results indicate that the barrier for rotation is higher than in the parent imines. Formation Iminium cations are obtained by protonation and alkylation of imines: : : They also are generated by the condensation of secondary amines with ketones or aldehydes: : This rapid, reversible reaction is one st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
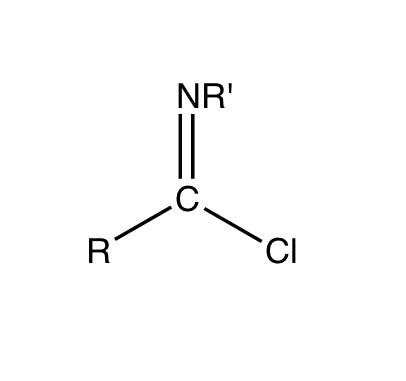
Imidoyl Chloride
Imidoyl chlorides are organic compounds that contain the functional group RC(NR')Cl. A double bond exist between the R'N and the carbon centre. These compounds are analogues of acyl chloride. Imidoyl chlorides tend to be highly reactive and are more commonly found as intermediates in a wide variety of synthetic procedures. Such procedures include Gattermann aldehyde synthesis, Houben-Hoesch reaction, Houben-Hoesch ketone synthesis, and the Beckmann rearrangement. Their chemistry is related to that of enamines and their tautomers when the α hydrogen is next to the C=N bond.Ulrich, H. The Chemistry of Imidoyl Halides; Plenum Press: New York, 1968; pp. 55–112. Many chlorinated N-heterocycles are formally imidoyl chlorides, e.g. 2-Chloropyridine, 2-chloropyridine, 2, 4, and 6-chloropyrimidines. Synthesis and properties Imidoyl halides are synthesized by combining amides and halogenating agents. The structure of the carboxylic acid amides plays a role in the outcome of the synthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |