|
Uranate
A uranate is a ternary oxide involving the element uranium in one of the oxidation states 4, 5 or 6. A typical chemical formula is MxUyOz, where M represents a cation. The uranium atom in uranates(VI) has two short collinear U–O bonds and either four or six more next nearest oxygen atoms. The structures are infinite lattice structures with the uranium atoms linked by bridging oxygen atoms. Uranium oxides are the foundation of the nuclear fuel cycle ("ammonium diuranate" and "sodium diuranate" are intermediates in the production of uranium oxide nuclear fuels) and their long-term geological disposal requires a thorough understanding of their chemical reactivity, phase transitions, and physical and chemical properties. Synthesis A method of general applicability involves combining two oxides in a high temperature reaction. For example, :Na2O + UO3 → Na2UO4 Another method is the thermal decomposition of a complex, such as an acetate complex. For example, microcrystalline bariu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Diuranate
Sodium diuranate, also known as the yellow oxide of uranium, is an inorganic chemical compound with the chemical formula . It is a sodium salt of a diuranate anion. It forms a hexahydrate . Sodium diuranate is commonly referred to by the initials SDU. Along with ammonium diuranate it was a component in early yellowcakes. The ratio of the two compounds is determined by process conditions; however, yellowcake is now largely a mix of uranium oxides. Preparation In the classical procedure for extracting uranium, pitchblende is broken up and mixed with sulfuric and nitric acids. The uranium dissolves to form uranyl sulfate and sodium carbonate is added to precipitate impurities. If the uranium in the ore is in the tetravalent oxidation state, an oxidiser is added to oxidise it to the hexavalent oxidation state, and sodium hydroxide is then added to make the uranium precipitate as sodium diuranate. The alkaline process of milling uranium ores involves precipitating sodium uran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Trioxide
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph is amorphous UO3. Production and use There are three methods to generate uranium trioxide. As noted below, two are used industrially in the reprocessing of nuclear fuel and uranium enrichment. # U3O8 can be oxidized at 500 °C with oxygen. Note that above 750 °C even in 5 atm O2 UO3 decomposes into U3O8. # Uranyl nitrate, UO2(NO3)2·6H2O can be heated to yield UO3. This occurs during the reprocessing of nuclear fuel. Fuel rods are dissolved in HNO3 to separate uranyl nitrate from plutonium and the fission products (the PUREX method). The pure uranyl nitrate is converted to solid UO3 by heating at 400 °C. After reduction with hydrogen (with other inert gas present) to uranium dioxide, the uranium can be used in new MOX fuel rod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Diuranate
Ammonium diuranate or (ADU) ((NH4)2U2O7), is one of the intermediate chemical forms of uranium produced during yellowcake production. The name "yellowcake" originally given to this bright yellow salt, now applies to mixtures of uranium oxides which are actually hardly ever yellow. It also is an intermediate in mixed-oxide ( MOX) fuel fabrication. Although it is usually called "ammonium diuranate" as though it has a "diuranate" ion , this is not necessarily the case. It can also be called diammonium diuranium heptaoxide. The structure was theorized to be similar to that of uranium trioxide dihydrate. Recent literature has shown that the structure more closely resembles the mineral metaschoepite, the partially dehydrated form of schoepite. It is precipitated by adding aqueous ammonium hydroxide after uranium extraction by tertiary amines in kerosene. This precipitate is then thickened and centrifuged before being calcined to uranium oxide. Canadian practice favours the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yellowcake
Yellowcake (also called urania) is a type of powdered uranium concentrate obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C. Overview Originally, raw uranium ore was extracted by traditional mining, and this is still the case in many mines. It is first crushed to a fine powder by passing it through crushers and grinders to produce "pulped" ore. This is further processed with concentrated acid, alkaline, or peroxide solutions to leach out the uranium. However, nearl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranyl
The uranyl ion with the chemical formula has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen, with uranium in the oxidation state +6. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complex (chemistry), complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing. Structure and bonding The uranyl ion is linear and symmetrical, specifically belonging to the D∞h point group, with both U–O bond lengths of about 180 pm. The bond lengths are indicative of the presence of multiple bonding between the uranium and oxygen atoms. Since uranium(VI) has the electronic configuration of the preceding noble gas, radon, the electrons used in forming the U–O bonds are supplied by the oxygen atoms. The electrons are donated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactive decay, radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes of uranium, isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordial nuclide, primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few Parts-per notation#Parts-per expressions, parts per million in soil, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Glass Collection
Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. Many contemporary u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fuel
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other atomic nucleus, nuclear devices to generate energy. Oxide fuel For fission reactors, the fuel (typically based on uranium) is usually based on the metal oxide; the oxides are used rather than the metals themselves because the oxide melting point is much higher than that of the metal and because it cannot burn, being already in the oxidized state. Uranium dioxide Uranium dioxide is a black semiconductor, semiconducting solid. It can be made by heating uranyl nitrate to form . : This is then converted by heating with hydrogen to form UO2. It can be made from Enriched uranium, enriched uranium hexafluoride by reacting with ammonia to form a solid called ammonium diuranate, . This is then heated (Calcination, calcined) to form and U3O8 which is then converted by heating with hydrogen or ammonia to form UO2. The UO2 is mixed with an organic binder and pressed in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Nitrate
Ammonium nitrate is a chemical compound with the formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, but does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, an industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices. Many countries are phasing out its use in consumer applications due to concerns over its potential for misuse.Ammonium nitrate sold by ton as U.S. regulati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
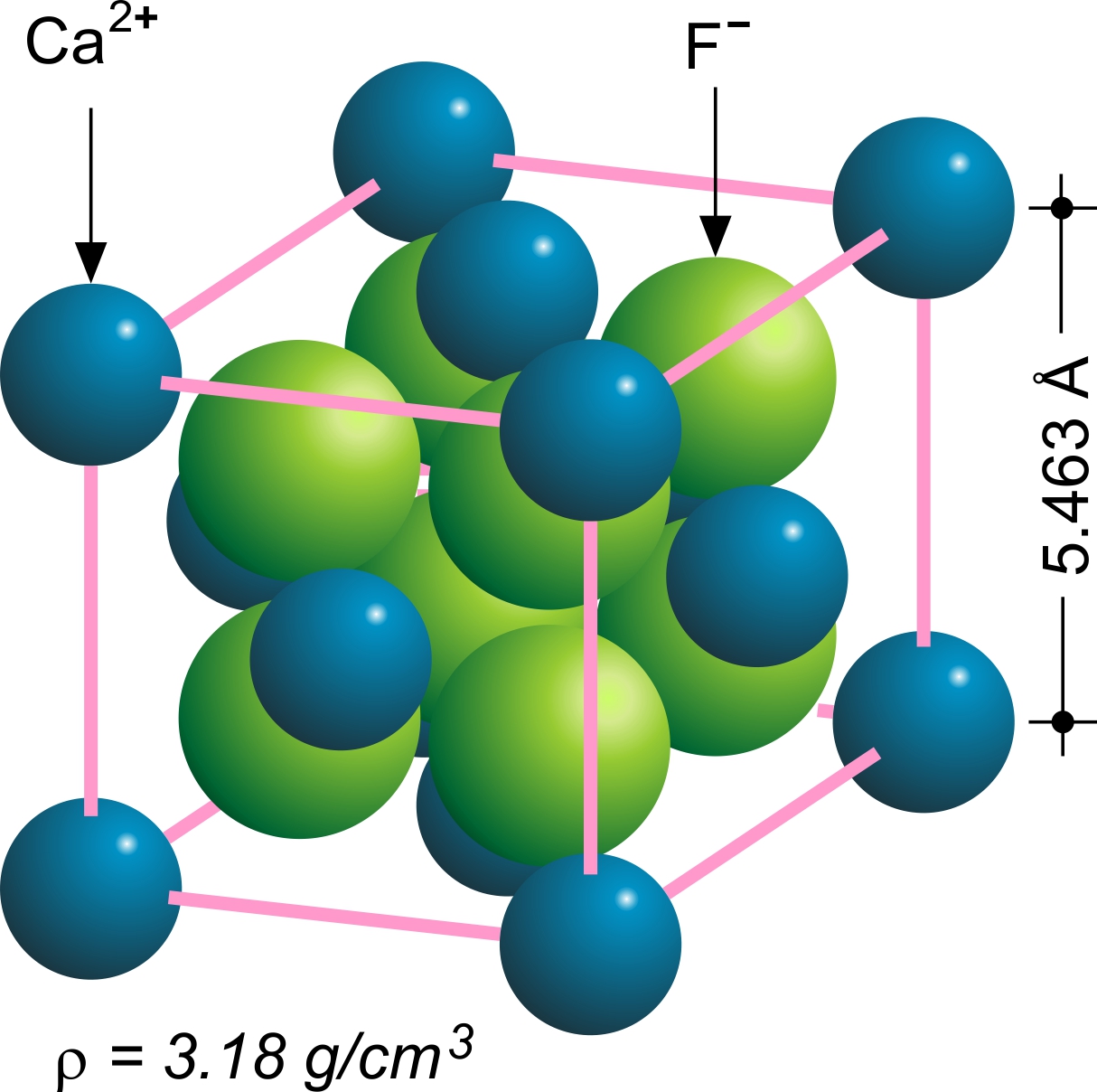
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |