|
Tributyltin Azide
Tributyltin azide is an organotin compound with the formula (C4H9)3SnN3. It is a colorless solid although older samples can appear as yellow oils. The compound is used as a reagent in organic synthesis. Synthesis and reactions Tributyltin azide is synthesized by the salt metathesis reaction of tributyltin chloride and sodium azide. It is a reagent used in the synthesis of tetrazoles, which in turn are used to generate angiotensin II receptor antagonists. In some applications, tributyltin azide has been replaced by the less toxic trioctyltin azide and organoaluminium azides. Safety Lower alkyl In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ... tin compounds are often highly toxic and have penetrating odors. Tributyltin azide causes skin rashes, itching or blisters.{{cite jour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MmHg
A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high, and currently defined as exactly pascals. It is denoted mmHg or mm Hg. Although not an SI unit, the millimetre of mercury is still routinely used in medicine, meteorology, aviation, and many other scientific fields. One millimetre of mercury is approximately 1 Torr, which is of standard atmospheric pressure ( ≈ ). Although the two units are not equal, the relative difference (less than ) is negligible for most practical uses. History For much of human history, the pressure of gases like air was ignored, denied, or taken for granted, but as early as the 6th century BC, Greek philosopher Anaximenes of Miletus claimed that all things are made of air that is simply changed by varying levels of pressure. He could observe water evaporating, changing to a gas, and felt that this applied even to solid matter. More cond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
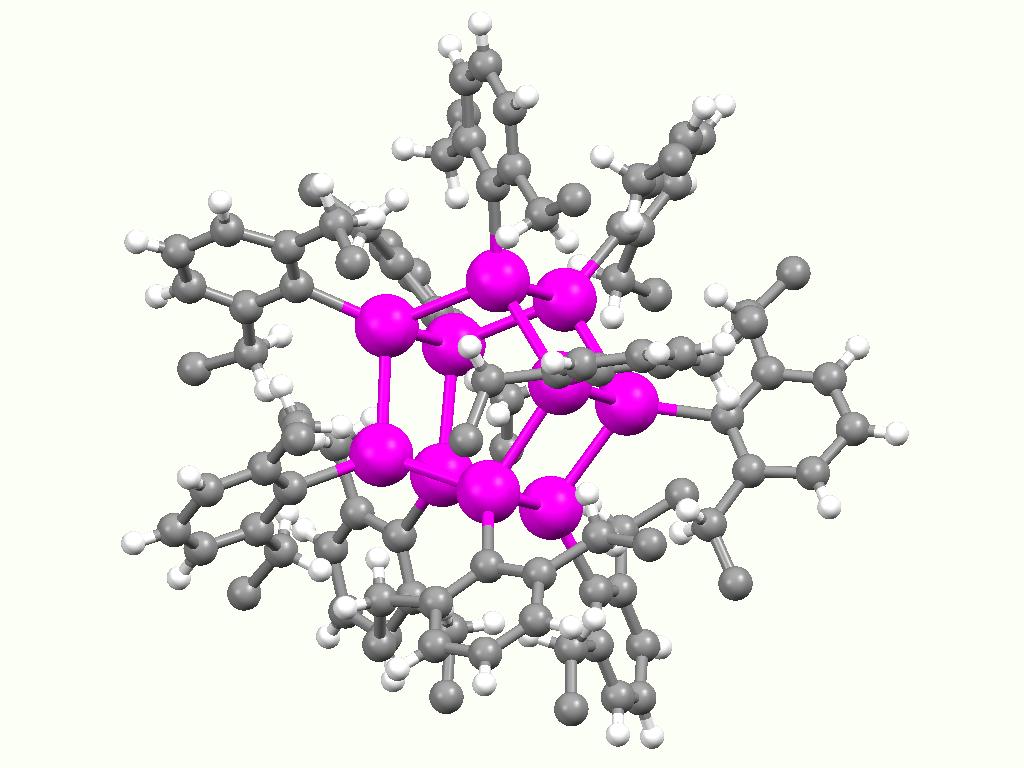
Organotin Compound
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known but less important. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt Metathesis Reaction
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme: :AB + CD -> AD + CB The bond between the reacting species can be either ionic or covalent. Classically, these reactions result in the precipitation of one product. In older literature, the term double decomposition is frequently encountered. The term double decomposition is more specifically used when at least one of the substances does not dissolve in the solvent, as the ligand or ion exchange takes place in the solid state of the reactant. For example: :AX(aq) + BY(s) → AY(aq) + BX(s). Types of reactions Counterion exchange Salt metathesis is a common technique for exchanging counterions. The choice of reactants is guided by a solubility chart or lattice energy. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributyltin Chloride
Tributyltin chloride is an organotin compound with the formula ( C4H9)3SnCl. It is a colorless liquid that is soluble in organic solvents. Preparation and reactions The compound is prepared by a redistribution reaction by combining stannic chloride and tetrabutyltin: :3 (C4H9)4Sn + SnCl4 → 4 (C4H9)3SnCl Tributyltin chloride hydrolyzes to the oxide C4H9)3Snsub>2O Tributyltin chloride is used as a precursor to other organotin compounds{{cite journal, title=Palladium-catalyzed Coupling Of Acid Chlorides With Organotin Reagents: Ethyl (E)-4-(4-nitrophenyl)-4-oxo-2-butenoate, authors=A. F. Renaldo, J. W. Labadie, And J. K. Stille, journal=Org. Synth., year=1989, volume=67, page=86, doi=10.15227/orgsyn.067.0086 and reagents, such as tributyltin hydride Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. Synthesis an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated. Structure and bonding Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1''H''-, 2''H''-, and 5''H''-tetrazole. The 1''H''- and 2''H''- isomers are tautomers, with the equilibrium lying on the side of 1''H''-tetrazole in the solid phase. In the gas phase, 2''H''-tetrazole dominates. These isomers can be regarded as aromatic, with 6 π-electrons, while the 5''H''-isomer is nonaromatic. Synthesis 1''H''-Tetrazole was first prepared by the reaction of anhydrous hydrazoic acid and hydrogen cyanide under pressure. Treatment of organic nitriles with sodium azide in the presence of iodine or silica-supported sodium bisulfate as a heterogeneous catalyst enables an advantageous synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angiotensin II Receptor Antagonists
Angiotensin II receptor blockers (ARBs), formally angiotensin II receptor type 1 (AT1) antagonists, also known as angiotensin receptor blockers, angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals that bind to and inhibit the angiotensin II receptor type 1 (AT1) and thereby block the arteriolar contraction and sodium retention effects of renin–angiotensin system. Their main uses are in the treatment of hypertension (high blood pressure), diabetic nephropathy (kidney damage due to diabetes) and congestive heart failure. They ''selectively'' block the activation of the AT1 receptor, preventing the binding of angiotensin II compared to ACE inhibitors. ARBs and the similar-attributed ACE inhibitors are both indicated as the first-line antihypertensives in patients developing hypertension along with left-sided heart failure. However, ARBs appear to produce less adverse effects compared to ACE inhibitors. Medical uses Angiotensin II ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoaluminium
Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins. History The first organoaluminium compound (C2H5)3Al2I3 was discovered in 1859. Organoaluminium compounds were, however, little known until the 1950s when Karl Ziegler and colleagues discovered the direct synthesis of trialkylaluminium compounds and applied these compounds to catalytic olefin polymerization. This line of research ultimately resulted in the Nobel Prize to Ziegler. Structure and bonding Aluminium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compounds
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known but less important. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |