|
Tetra-n-butylammonium Iodide
Tetra-''n''-butylammonium iodide (TBAI) is a quaternary ammonium salt with an iodide counterion. It is used for synthesizing tetra-''n''-butylammonium triiodide by mixing with iodine. Properties The solid crystal of tetra-''n''-butylammonium iodide is in the monoclinic crystal system. It has space group ''C''2/''c''. The unit cell has dimensions a=14.2806 b=14.1864 c=19.5951 β=111.149. There are eight formulae in the unit cell (Z=8), which has volume 3702.4 Å3. The enthalpy of formation ΔfH0 of tetra-''n''-butylammonium iodide is −499 kJ/mol, which is lower than that for the bromide or chloride (−540, −564 kJ/mol). At lower temperatures with water tetra-''n''-butylammonium iodide forms a clathrate hydrate. The tetra-''n''-butylammonium cation is large and hydrophobic. The absolute enthalpy of hydration (from gas phase) is −260 kJ/mol. The He(I) photoelectron spectrum of tetra-''n''-butylammonium iodide contains a peak at 11 eV due to the te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabutylammonium Bromide
Tetrabutylammonium bromide (TBAB) is a quaternary ammonium salt with a bromide commonly used as a phase transfer catalyst. It is used to prepare many other tetrabutylammonium salts by salt metathesis reactions. The anhydrous form is a white solid. In addition to being cheap, tetrabutylammonium bromide is also environmentally friendly, has a greater degree of selectivity, is operationally simple, non-corrosive, and can be recycled easily as well. Preparation and reactions Tetrabutylammonium bromide can be prepared by the alkylation of tributylamine with 1-bromobutane. Tetrabutylammonium bromide is used to prepare other salts of the tetrabutylammonium cation by salt metathesis reactions., , ;. It serves as a source of bromide ions for substitution reactions. It is one of a commonly-used phase transfer catalyst. As its melting point is just over 100 °C and decreases in the presence of other reagents, it can be considered an ionic liquid. Role in semi-clathrate formation TBAB ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabutylammonium Chloride
Tetrabutylammonium chloride is the organic compound with the formula (C4H9)4NCl. A white water-soluble solid, it is a quaternary ammonium salt of chloride. It is a precursor to other tetrabutylammonium salts. Often tetrabutylammonium bromide Tetrabutylammonium bromide (TBAB) is a quaternary ammonium salt with a bromide commonly used as a phase transfer catalyst. It is used to prepare many other tetrabutylammonium salts by salt metathesis reactions. The anhydrous form is a white soli ... is preferred as a source of tetrabutylammonium because it is less hygroscopic than the chloride. References Tetrabutylammonium salts Chlorides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabutylammonium Fluoride
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tetrahydrofuran. TBAF is used as a source of fluoride ion in organic solvents. Preparation and properties TBAF can be prepared by passing hydrofluoric acid through an ion-exchange resin, followed by tetrabutylammonium bromide. Upon evaporation of the water, TBAF can be collected as an oil in quantitative yield. Preparing anhydrous samples is of interest as the basicity of fluoride increases by more than 20 p''K'' units on passing from aqueous to aprotic solvent. However, heating samples of the hydrated material to 77 °C under vacuum causes decomposition to the hydrogen difluoride salt. Similarly, samples dried at 40 °C under high vacuum still contain 10-30 mol% of water and some 10% of difluoride. Instead, anhydrous TBAF ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabutylammonium Hydroxide
Tetrabutylammonium hydroxide is the chemical compound with the formula (C4H9)4NOH, abbreviated Bu4NOH with the acronym TBAOH or TBAH. This species is employed as a solution in water or alcohols. It is a common base in organic chemistry. Relative to more conventional inorganic bases, such as KOH and NaOH, Bu4NOH is more soluble in organic solvents.. Preparation and reactions Solutions of Bu4NOH are usually prepared ''in situ'' from butylammonium halides, Bu4NX, for example by reacting them with silver oxide or using an ion exchange resin. Attempts to isolate Bu4NOH induces Hofmann elimination, leading to Bu3N and 1-butene. Solutions of Bu4NOH are typically contaminated with Bu3N for this reason. Treatment of Bu4NOH with a wide range of acids gives water and the other tetrabutylammonium salts: Bu4NOH + HX -> Bu4NX + H2O Applications Bu4NOH is a strong base that is used often under phase-transfer conditions to effect alkylations and deprotonations. Typical reactions include benz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Salt
In chemistry, quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure , R being an alkyl group or an aryl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis Quaternary ammonium comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Counterion
160px, Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.">cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion. In chemistry, a counterion (sometimes written as "counter ion", pronounced as such) is the ion that accompanies an Ionic compound, ionic species in order to maintain Electric charge, electric neutrality. In table salt (NaCl, also known as sodium chloride) the sodium ion (positively charged) is the counterion for the chloride ion (negatively charged) and vice versa. A counterion will be more commonly referred to as an anion or a cation, depending on whether it is negatively or positively charged. Thus, the counterion to an anion will be a cation, and vice versa. In biochemistry, counterions are generally vaguely defined. Depending on their charge, proteins are associated with a variety of smaller ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabutylammonium Triiodide
Tetra-''n''-butylammonium triiodide (TBAI3) is a quaternary ammonium salt with a triiodide counterion. It is a common carrier of the triiodide used in chemical synthesis of photovoltaic materials, organic conductors and superconductors. In crystals, the triiodide moieties are linear and shows high crystallinity.{{Cite journal , last1=Brotherton , first1=Wendy S. , last2=Clark , first2=Ronald J. , last3=Zhu , first3=Lei , date=2012-08-03 , title=Synthesis of 5-Iodo-1,4-disubstituted-1,2,3-triazoles Mediated by in Situ Generated Copper(I) Catalyst and Electrophilic Triiodide Ion , url=https://doi.org/10.1021/jo300841c , journal=The Journal of Organic Chemistry , volume=77 , issue=15 , pages=6443–6455 , doi=10.1021/jo300841c , pmid=22780866 , issn=0022-3263 The crystals have a black appearance with a needle or plate-like habit. See also * Triiodide * Tetrabutylammonium tribromide * Organic superconductor An organic superconductor is a synthetic organic compound that exhibits s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal classes The table below org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
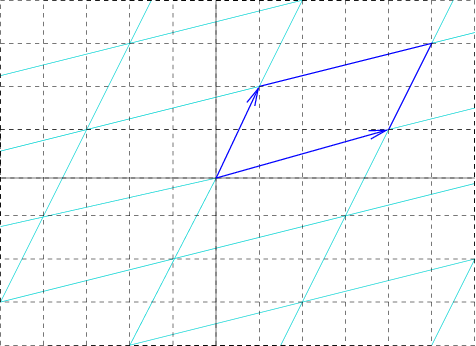
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |