|
Terconazole
Terconazole is an antifungal drug used to treat vaginal yeast infection. It comes as a lotion or a suppository and disrupts the biosynthesis of fats in a yeast cell. It has a relatively broad spectrum compared to azole compounds but not triazole compounds. Testing shows that it is a suitable compound for prophylaxis for those that suffer from chronic vulvovaginal candidiasis. Medical uses Terconazole is approved to treat vulvovaginal candidiasis (vaginal thrush). It works as a broad spectrum antifungal and has shown to be an effective first-line treatment against other ''Candida'' species. It also shows effectiveness against dermatomycoses in animal models. A review found that short-term rates for intravaginally administered azole treatments shows cure in 80% of cases in a short term follow-up and 66% over long term follow-up. In a double-blind study by Slavin in 1992, terconazole showed a 75% mycological cure over a short-term period (7–14 days) and 100% mycological cure over ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terconazole Synthesis
Terconazole is an antifungal drug used to treat vaginal yeast infection. It comes as a lotion or a suppository and disrupts the biosynthesis of fats in a yeast cell. It has a relatively broad spectrum compared to azole compounds but not triazole compounds. Testing shows that it is a suitable compound for prophylaxis for those that suffer from chronic vulvovaginal candidiasis. Medical uses Terconazole is approved to treat vulvovaginal candidiasis (vaginal thrush). It works as a broad spectrum antifungal and has shown to be an effective first-line treatment against other ''Candida'' species. It also shows effectiveness against dermatomycoses in animal models. A review found that short-term rates for intravaginally administered azole treatments shows cure in 80% of cases in a short term follow-up and 66% over long term follow-up. In a double-blind study by Slavin in 1992, terconazole showed a 75% mycological cure over a short-term period (7–14 days) and 100% mycological cure over ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifungal
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually yes obtained by a doctor's prescription, but a few are available over the counter (OTC). Types of antifungal There are two types of antifungals: local and systemic. Local antifungals are usually administered topically or vaginally, depending on the condition being treated. Systemic antifungals are administered orally or intravenously. Of the clinically employed azole antifungals, only a handful are used systemically. These include ketoconazole, itraconazole, fluconazole, fosfluconazole, voriconazole, posaconazole, and isavuconazole. Examples of non-azole systemic antifungals include griseofulvin and terbinafine. Classes Polyenes A polyene is a molecule with multiple c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast plant steroids are produced via cycloartenol. Role in biosynthesis of other steroids Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by cytochrome P450, CYP51 eventually yields cholesterol. Biosynthesis Research Lanosterol has been identified as a key component in maintaining eye lens clarity. Pre-clinical research has identified Lanosterol as a possible agent for the reversal and prevention of cataracts. In vivo experiments on dogs showed significant reversal of cataracts within 6 weeks of lanosterol injection. In 2018, Lanosterol was shown to improve lens clarity in cells with lens clouding due to aging or physical stressors. A subsequent study found positive results on the optics of the lens in mice with cataracts (Wang, Hoshino,Uesugi, Yagi, Pierscionek and Andley (2022). Use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Compounds
In organic chemistry, propyl is a three- carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it bein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
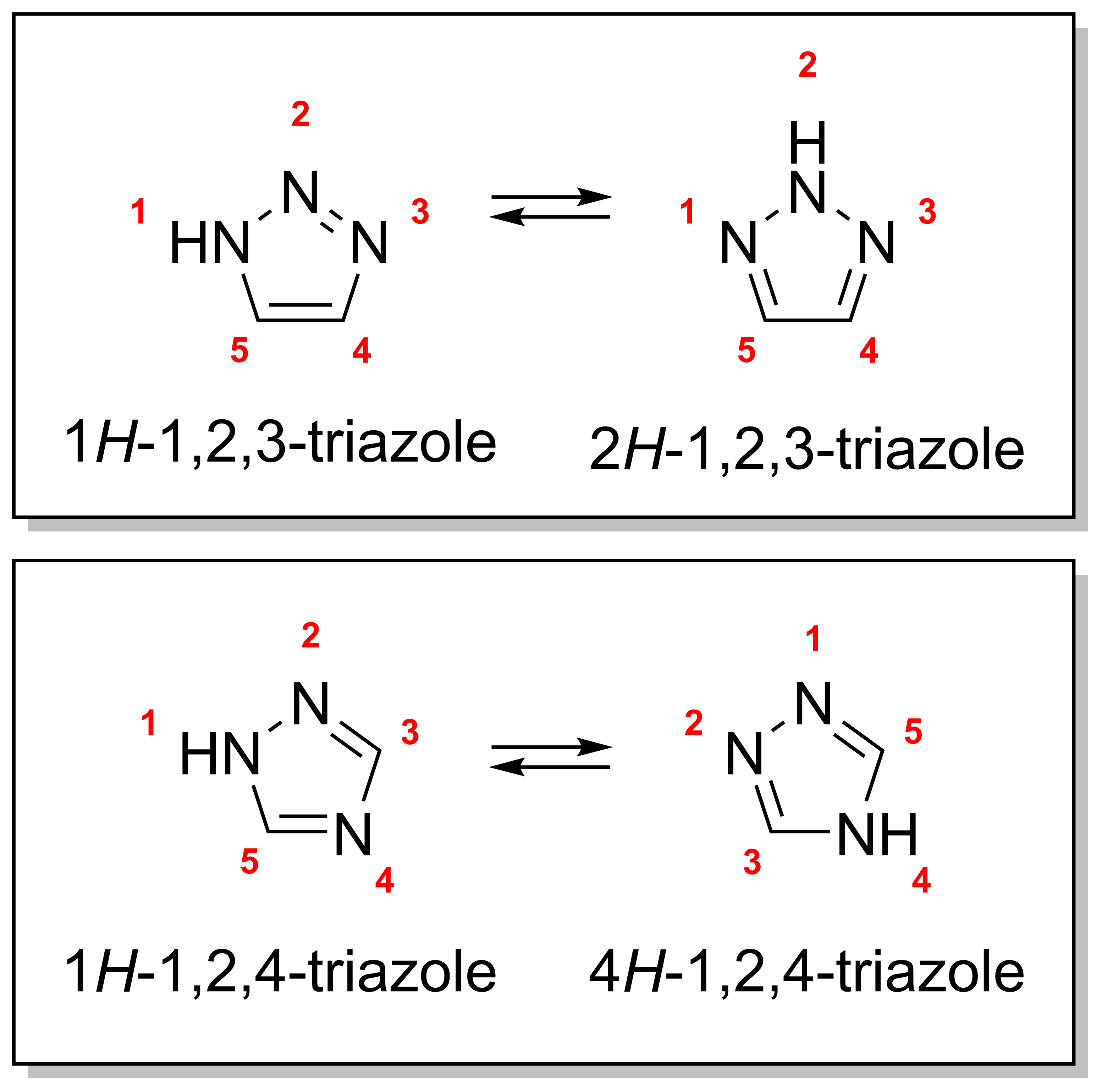
Triazole Antifungals
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring. Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as haptic ligands. Isomerism There are four triazole isomers, which are conventionally divided into two pairs of tautomers. In the 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-triazoles, an interstitial carbon separates out one nitrogen atom. Each category has two tautomers that differ by which nitrogen has a hydrogen bonded to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Ethers
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanosterol 14α-demethylase Inhibitors
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast plant steroids are produced via cycloartenol. Role in biosynthesis of other steroids Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol. Biosynthesis Research Lanosterol has been identified as a key component in maintaining eye lens clarity. Pre-clinical research has identified Lanosterol as a possible agent for the reversal and prevention of cataracts. In vivo experiments on dogs showed significant reversal of cataracts within 6 weeks of lanosterol injection. In 2018, Lanosterol was shown to improve lens clarity in cells with lens clouding due to aging or physical stressors. A subsequent study found positive results on the optics of the lens in mice with cataracts (Wang, Hoshino,Uesugi, Yagi, Pierscionek and Andley (2022). Use Lanosterol i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
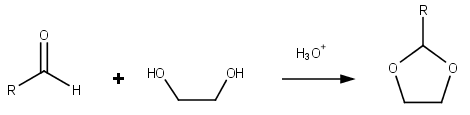
Dioxolanes
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals. As a class of compounds Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol. (+)-''cis''-Dioxolane is the trivial name for which is a muscarinic acetylcholine receptor agonist. Protecting groups Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection of carbonyls including as dioxolanes are kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Janssen Pharmaceutica
Janssen Pharmaceuticals is a pharmaceutical company headquartered in Beerse, Belgium, and wholly-owned by Johnson & Johnson. It was founded in 1953 by Paul Janssen. In 1961, Janssen Pharmaceuticals was purchased by New Jersey-based American corporation Johnson & Johnson, and became part of Johnson & Johnson Pharmaceutical Research and Development (J&J PRD), now renamed to Janssen Research and Development (JRD), which conducts research and development activities related to a wide range of human medical disorders, including mental illness, neurological disorders, anesthesia and analgesia, gastrointestinal disorders, fungal infection, HIV/AIDS, allergies and cancer. Janssen and Ortho-McNeil Pharmaceutical have been placed in the Ortho-McNeil-Janssen group within Johnson & Johnson Company. Subsidiaries * Actelion * Cilag AG * Janssen Biotech (formerly ''Centocor'') * Janssen Vaccines (formerly ''Crucell'') * Tibotec * Beijing Dabao Cosmetics Co., Ltd. History The early ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketoconazole
Ketoconazole, sold under the brand name Nizoral among others, is an antiandrogen and antifungal medication used to treat a number of fungal infections. Applied to the skin it is used for fungal skin infections such as tinea, cutaneous candidiasis, pityriasis versicolor, dandruff, and seborrheic dermatitis. Taken by mouth it is a less preferred option and only recommended for severe infections when other agents cannot be used. Other uses include treatment of excessive male-patterned hair growth in women and Cushing's syndrome. Common side effects when applied to the skin include redness. Common side effects when taken by mouth include nausea, headache, and liver problems. Liver problems may result in death or the need for a liver transplantation. Other severe side effects when taken by mouth include QT prolongation, adrenocortical insufficiency, and anaphylaxis. It is an imidazole and works by hindering the production of ergosterol required for the fungal cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphotericin B
Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis. For certain infections it is given with flucytosine. It is typically given intravenously (injection into a vein). Common side effects include a reaction with fever, chills, and headaches soon after the medication is given, as well as kidney problems. Allergic symptoms including anaphylaxis may occur. Other serious side effects include low blood potassium and myocarditis (inflammation of the heart). It appears to be relatively safe in pregnancy. There is a lipid formulation that has a lower risk of side effects. It is in the polyene class of medications and works in part by interfering with the cell membrane of the fungus. Amphotericin B was isolated from ''Streptomyces nodosus'' in 1955 at the Squibb For Medical Research Ins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |