|
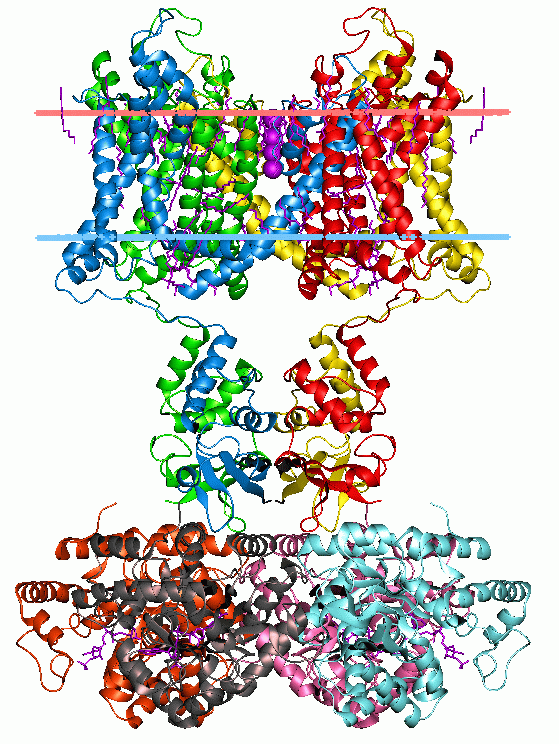
T4 Holin
The T4 Holin FamilyTC# 1.E.8 is a group of putative pore-forming proteins that does not belong to one of the seven holin superfamilies. T-even phage such as T4 use a holin-endolysin system for host cell lysis. Although the endolysin of phage T4 encoded by the e gene (Lysozyme E) was identified in 1961, the holin (product of gene t and called T-holin) was not characterized until 2001. A representative list of proteins belonging to the T4 holin family can be found in thTransporter Classification Database Structure T4 holin is fairly large, about 218 amino acyl residues (aas) in length. The protein is highly hydrophilic with 49 acidic and basic residues distributed along its length and a single putative transmembrane segment (TMS) near its N-terminus, leaving most of the protein in the periplasm. Function The large periplasmic domain is a major determinant in the timing mechanism and is involved in lysis inhibition (LIN). LIN involves the antiholin rI protein of T4 (SeTC# 1.E.8.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysis
Lysis ( ) is the breaking down of the membrane of a cell, often by viral, enzymic, or osmotic (that is, "lytic" ) mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a ''lysate''. In molecular biology, biochemistry, and cell biology laboratories, cell cultures may be subjected to lysis in the process of purifying their components, as in protein purification, DNA extraction, RNA extraction, or in purifying organelles. Many species of bacteria are subject to lysis by the enzyme lysozyme, found in animal saliva, egg white, and other secretions. Phage lytic enzymes (lysins) produced during bacteriophage infection are responsible for the ability of these viruses to lyse bacterial cells. Penicillin and related β-lactam antibiotics cause the death of bacteria through enzyme-mediated lysis that occurs after the drug causes the bacterium to form a defective cell wall. If the cell wall is completely lost and the penicillin was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have structures that are either simple or elaborate. Their genomes may encode as few as four genes (e.g. MS2) and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into its cytoplasm. Bacteriophages are among the most common and diverse entities in the biosphere. Bacteriophages are ubiquitous viruses, found wherever bacteria exist. It is estimated there are more than 1031 bacteriophages on the planet, more than every other organism on Earth, including bacteria, combined. Viruses are the most abundant biological entity in the water column of the world's oceans, and the second largest component o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periplasm
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the ''periplasmic space'' in gram-negative bacteria. Using cryo-electron microscopy it has been found that a much smaller periplasmic space is also present in gram-positive bacteria., Matias, V. R., and T. J. Beveridge. 2005. Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space. Mol. Microbiol. 56:240-251. ., Zuber B, Haenni M, Ribeiro T, Minnig K, Lopes F, Moreillon P, Dubochet J. 2006. Granular layer in the periplasmic space of Gram-positive bacteria and fine structures of Enterococcus gallinarum and Streptococcus gordonii septa revealed by cryo-electron microscopy of vitreous sections. J Bacteriol. 188:6652-6660. The periplasm may constitute up to 40% of the total cell volume of gram-negative bacteria, but is a much smaller percentage in gram-positive bacte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysin
Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternative mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins. Lysins are being used as antibacterial agents due to their high effectiveness and specificity in comparison with antibiotics, which are susceptible to bacterial resistance. Structure Double-stranded DNA phage lysins tend to lie within the 25 t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holin
Holins are a diverse group of small proteins produced by dsDNA bacteriophages in order to trigger and control the degradation of the host's cell wall at the end of the lytic cycle. Holins form pores in the host's cell membrane, allowing lysins to reach and degrade peptidoglycan, a component of bacterial cell walls. Holins have been shown to regulate the timing of lysis with great precision. Over 50 unrelated gene families encode holins, making them the most diverse group of proteins with common function. Together with lysins, holins are being studied for their potential use as antibacterial agents. While canonical holins act by forming large pores, pinholins such as the S protein of lambdoid phage 21 act by forming heptameric channels that depolarize the bacterial membrane. They are associated with SAR endolysins, which remain inactive in the periplasm prior to the depolarization of the membrane. Viruses that infect eukaryotic cells may use similar channel-forming proteins calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactococcus Lactis
''Lactococcus lactis'' is a Gram-positive bacterium used extensively in the production of buttermilk and cheese, but has also become famous as the first genetically modified organism to be used alive for the treatment of human disease. ''L. lactis'' cells are cocci that group in pairs and short chains, and, depending on growth conditions, appear ovoid with a typical length of 0.5 - 1.5 µm. ''L. lactis'' does not produce spores ( nonsporulating) and are not motile ( nonmotile). They have a homofermentative metabolism, meaning they produce lactic acid from sugars. They've also been reported to produce exclusive L-(+)-lactic acid. However, reported D-(−)-lactic acid can be produced when cultured at low pH. The capability to produce lactic acid is one of the reasons why ''L. lactis'' is one of the most important microorganisms in the dairy industry. Based on its history in food fermentation, ''L. lactis'' has generally recognized as safe (GRAS) status, with few case reports o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holin
Holins are a diverse group of small proteins produced by dsDNA bacteriophages in order to trigger and control the degradation of the host's cell wall at the end of the lytic cycle. Holins form pores in the host's cell membrane, allowing lysins to reach and degrade peptidoglycan, a component of bacterial cell walls. Holins have been shown to regulate the timing of lysis with great precision. Over 50 unrelated gene families encode holins, making them the most diverse group of proteins with common function. Together with lysins, holins are being studied for their potential use as antibacterial agents. While canonical holins act by forming large pores, pinholins such as the S protein of lambdoid phage 21 act by forming heptameric channels that depolarize the bacterial membrane. They are associated with SAR endolysins, which remain inactive in the periplasm prior to the depolarization of the membrane. Viruses that infect eukaryotic cells may use similar channel-forming proteins calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein famil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Proteins
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the sur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |