|
Tris
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2. It is extensively used in biochemistry and molecular biology as a component of buffer solutions such as in TAE and TBE buffers, especially for solutions of nucleic acids. It contains a primary amine and thus undergoes the reactions associated with typical amines, e.g., condensations with aldehydes. Tris also complexes with metal ions in solution. In medicine, tromethamine is occasionally used as a drug, given in intensive care for its properties as a buffer for the treatment of severe metabolic acidosis in specific circumstances. Some medications are formulated as the "tromethamine salt" including Hemabate ( carboprost as trometamol salt), and " ketorolac trometamol". In 2023 a strain of ''Pseudomonas hunanensis'' was found to be able to degrade TRIS buffer. Since Tris' pKa is more strongly temperature dependent, its use is not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TAE Buffer
TAE buffer is a buffer solution containing a mixture of Tris base, acetic acid and EDTA. In molecular biology, it is used in agarose electrophoresis typically for the separation of nucleic acids such as DNA and RNA. It is made up of Tris-acetate buffer, usually at pH 8.3, and EDTA, which sequesters divalent cations. TAE has a lower buffer capacity than TBE buffer, TBE and can easily become exhausted, but linear, double stranded DNA runs faster in TAE. According to studies by Brody and Kern, sodium boric acid is a superior and cheaper conductive media for most DNA gel electrophoresis applications. Uses TAE (Tris-acetate-EDTA) buffer is used as both a running buffer and in agarose gels. Its use in denaturing gradient gel electrophoresis methods for broad-range mutation analysis has also been described. TAE has been used at various concentrations to study the mobility of DNA in solution with and without sodium chloride. However, high concentrations of sodium chloride (and many othe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TBE Buffer
TBE or Tris/Borate/EDTA, is a buffer solution containing a mixture of Tris base, boric acid and EDTA. In molecular biology, TBE and TAE buffers are often used in procedures involving nucleic acids, the most common being electrophoresis. Tris-acid solutions are effective buffers for slightly basic conditions, which keep DNA deprotonated and soluble in water. EDTA is a chelator of divalent cations, particularly of magnesium (Mg2+). As these ions are necessary co-factors for many enzymes, including contaminant nucleases, the role of the EDTA is to protect the nucleic acids against enzymatic degradation. But since Mg2+ is also a co-factor for many useful DNA-modifying enzymes such as restriction enzymes and DNA polymerases, its concentration in TBE or TAE buffers is generally kept low (typically at around 1 mM). According to studies by Brody and Kern, sodium boric acid is a superior and cheaper conductive media for most DNA gel electrophoresis applications. Recipe (1 liter of 5X s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MES (buffer)
MES (2-(''N''-morpholino)ethanesulfonic acid) is a chemical compound that contains a morpholine ring. It has a molecular weight of 195.2 g/mol and the chemical formula is C6H13NO4S. Synonyms include: 2-morpholinoethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; 2-(''N''-morpholino)ethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; MES; MES hydrate; and morpholine-4-ethanesulfonic acid hydrate. MOPS is a similar pH buffering compound which contains a propanesulfonic moiety instead of an ethanesulfonic one. Applications MES is used as a buffering agent in biology and biochemistry. It has p''K''a value of 6.15 at 20 °C. The pH (and p''K''a at ionic strength I≠0) of the buffer solution changes with concentration and temperature, and this effect may be predicted using online calculators. MES is highly soluble in water. The melting point is approx. 300 °C. MES was developed as one of Good's buffers in the 1960s. These buffers were developed with the follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MOPS
MOPS (3-(''N''-morpholino)propanesulfonic acid) is a buffer solution, buffer introduced in the 1960s, one of the twenty Good's buffers. It is a structural analog to MES (buffer), MES, and like MES, its structure contains a morpholine ring. HEPES is a similar pH buffering compound that contains a piperazine ring. With a pKa, p''K''a of 7.20, MOPS is an excellent buffer for many biological systems at near-neutral pH. Applications MOPS is frequently used as a buffering agent in biology and biochemistry. It has been tested and recommended for polyacrylamide gel electrophoresis. Usage above 20 mM in mammalian cell culture work is not recommended. MOPS buffer solutions become discolored (yellow) over time, but reportedly slight discoloration does not significantly affect the buffering characteristics. See also *CAPS (buffer), CAPS *HEPPS (molecule), HEPPS *Tris *Buffer_solution#Common_buffer_compounds_used_in_biology, Common buffer compounds used in biology References {{reflist Exte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Standard
A primary standard in metrology is a standard that is sufficiently accurate such that it is not calibrated by or subordinate to other standards. Primary standards are defined via other quantities like length, mass and time. Primary standards are used to calibrate other standards referred to as working standards.Skoog, Douglas A., Donald M. West and F. James Holler. "Fundamentals of Analytical Chemistry 8th ed." Harcourt Brace College Publishers. 1995 ''Holt Science and Technology: Physical Science''. Ed. Rinehart and Winston, Inc. Holt. Holt McDougal (July 2000). . See Hierarchy of Standards. In chemistry Standards are used in analytical chemistry. Here, a primary standard is typically a reagent which can be weighed easily, and which is so pure that its weight is truly representative of the number of moles of substance contained. Features of a primary standard include: # High purity # Stability (low reactivity) # Low hygroscopicity (to minimize weight changes due to humidity) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
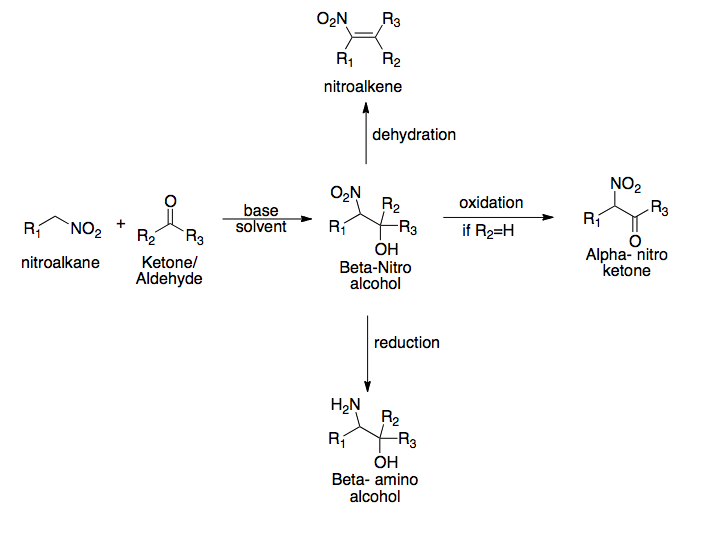
Henry Reaction
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction (nitroalkane, aldehyde, and alcohol). It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols" (aldehyde and alcohol). The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HEPES
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is a zwitterionic sulfonic acid buffering agent. It is one of the twenty Good's buffers. HEPES is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration (produced by aerobic respiration) when compared to bicarbonate buffers, which are also commonly used in cell culture. Lepe-Zuniga ''et al.'' reported an unwanted photochemical process wherein HEPES catalyzes a reaction with riboflavin when exposed to ambient light to produce hydrogen peroxide. This is not a problem in bicarbonate-based cell culture buffers. It is therefore strongly advised to keep solutions containing both HEPES and riboflavin in darkness as much as possible to prevent oxidation. HEPES has the following characteristics: * p''K''a1 (25 °C) = 3 * p''K''a2 (25 °C) = 7.5 * Useful pH range = 2.5 to 3.5 or 6.8 to 8.2 HEPES has negligible metal ion binding, making ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |