|
Trihalomethanes
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane () are replaced by halogen atoms. Trihalomethanes with all the same halogen atoms are called haloforms. Many trihalomethanes find uses in industry as solvents or refrigerants. Some THMs are also environmental pollutants, and a few are considered carcinogenic. Table of common trihalomethanes Industrial uses Only chloroform has significant applications of the haloforms. In the predominant application, chloroform is required for the production of tetrafluoroethylene (TFE), precursor to teflon. Chloroform is fluorinated by reaction with hydrogen fluoride to produce chlorodifluoromethane (R-22). Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE, with difluorocarbene as an intermediate. :CHCl3 + 2 HF -> CHClF2 + 2 HCl :2 CHClF2 -> C2F4 + 2 HCl Refrigerants and solvents Trihalomethanes released to the environment break down faster than chlorofluorocarbons ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and polytetrafluoroethylene (PTFE). Chloroform was once used as an inhalational anesthetic between the 19th century and the first half of the 20th century. It is miscible with many solvents but it is only very slightly soluble in water (only 8 g/L at 20°C). Structure and name The molecule adopts a tetrahedral molecular geometry with C3v symmetry. The chloroform molecule can be viewed as a methane molecule with three hydrogen atoms replaced with three chlorine atoms, leaving a single hydrogen atom. The name "chloroform" is a portmanteau of ''terchloride'' (tertiary chloride, a trichloride) and ''formyle'', an obsolete name for the methylylidene radical (CH) derived from formic acid. Natural occurrence Many kinds of seaweed produce chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromodichloromethane
Bromodichloromethane is a trihalomethane with formula . It is a colorless, nonflammable liquid which will dissolve in water, or evaporate in air. Most of the chemical is produced through the chlorine disinfection process, and as a result it can occur in municipally-treated drinking water. It is also produced in small quantities by algae in the oceans. According to the CDC, levels normal in drinking water are not known to cause health problems, but it has been classified by the US EPA as a probable human carcinogen. Bromodichloromethane has formerly been used as a flame retardant, and a solvent for fats and waxes and because of its high density for mineral separation. Now it is only used as a reagent or intermediate in organic chemistry. In the US it is only produced in small quantities, which are used for these chemical reasons. For example, it can be used to produce phenyl(bromodichloromethyl)mercury, which is widely used in the production of dichlorocarbene. It can be prepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguisher, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Difluorocarbene
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 ms, in solution and in the gas phase, respectively.Douglas A Jean Osteraas "Difluorocarbene Modification of Polymer and Fiber Surfaces," ''Journal of Applied Polymer''1969, volume 13, 1523-1535. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene, which is produced on an industrial scale as the precursor to Teflon (PTFE). Bonding in difluorocarbene In general, carbenes exist in either singlet or triplet states, which are often quite close in energy. Singlet carbenes have spin-paired electrons and a higher energy empty 2p orbital. In a triplet carbene, one electron occupies the hybrid orbital and the other is promoted to the 2p orbital.Jones. Maitland.''Organic Chemistry'', 3rd ed, W. W. Norton, 2005, 460-465. . For most carbenes, the triplet state is more stable than the corresponding singlet. In the case of fluorinated carbenes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
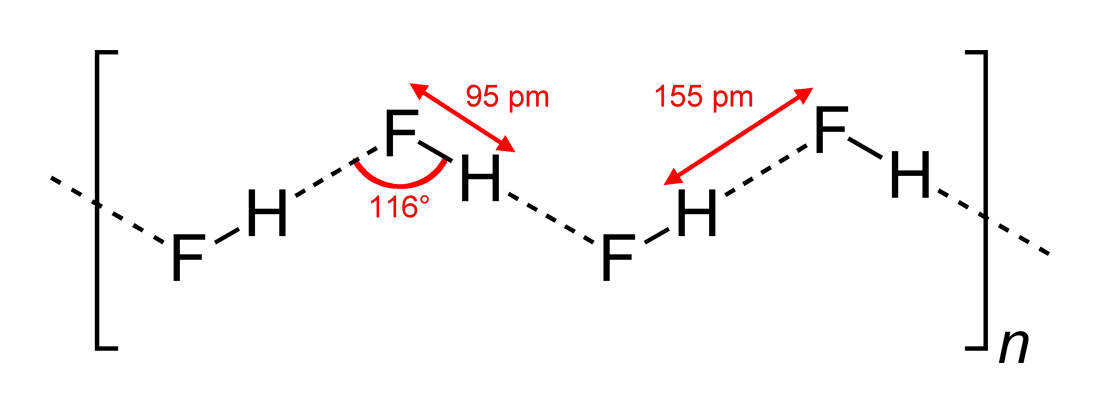
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high- molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. When used as a lubricant, PTFE reduces fric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroethylene
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula . It is a colorless gas. Its structure is . It is used primarily in the industrial preparation of fluoropolymers. It is the simplest perfluorinated alkene. It was first reported as "dicarbon tetrafluoride" in 1890. Properties Tetrafluoroethylene is a synthetic colorless, odorless gas that is insoluble in water. Like all unsaturated fluorocarbons, it is susceptible to nucleophilic attack. It is unstable towards decomposition to carbon and carbon tetrafluoride () and prone to form explosive peroxides in contact with air. Industrial use Polymerization of tetrafluoroethylene produces polytetrafluoroethylene (PTFE) polymers such as Teflon and Fluon. PTFE is one of the two fluorocarbon resins composed wholly of fluorine and carbon. The other resin composed purely of carbon and fluorine is the copolymer of TFE with typically 6–9% hexafluoropropene (HFP), which is known as FEP (fluorinated ethylene propylene c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoform
Iodoform (also known as triiodomethane) is the organoiodine compound with the chemical formula . It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant. Naming The name iodoform originates with the "formyle radical," an archaic term for the HC moiety, and is retained for historical consistency. A full, modern name is triiodomethane. Another possible name is "carbon hydride triiodide". The "hydride" in the latter is sometimes omitted, though the IUPAC recommends against doing so, as "carbon triiodide" could also mean (hexaiodoethane, a highly unstable compound). Structure The molecule adopts a tetrahedral molecular geometry, tetrahedral geometry with C3v symmetry group, symmetry. Synthesis and reactions The synthesis of iodofo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoform
Bromoform is an organic compound with the chemical formula . It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water (one part bromoform in 800 parts water) and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis Bromoform was discovered in 1832 by Löwig who distilled a mixture of bromal and potassium hydroxide, as analogous to preparation of chloroform from chloral. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium brom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |