|
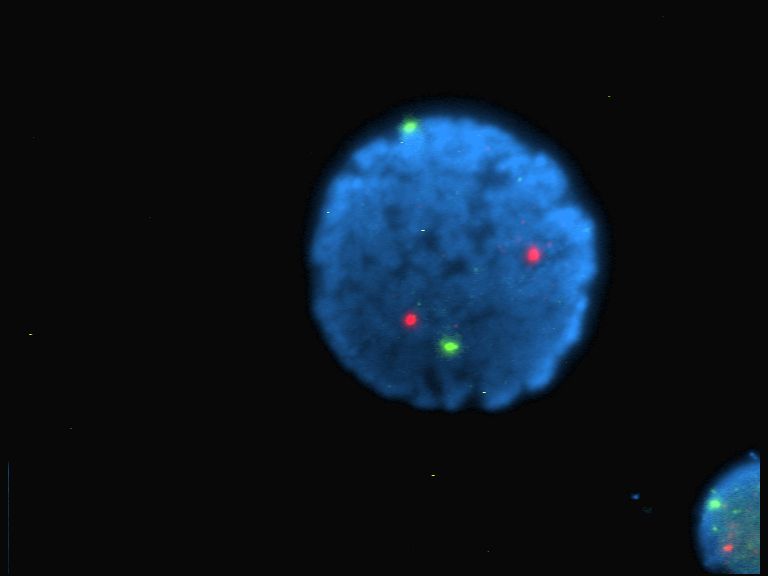
Titan Yellow
Titan yellow is a compound with formula C28H19N5Na2O6S4. It is a triazene dye used as a stain and fluorescent indicator in microscopy. Thiazole Yellow G at Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group.
Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and ... It is also used for the colorimetric indication of various compounds and is an acid-base indicator. As an acid-base indicator, it changes color from yellow to red between pH 12 and pH 13.
See also ...
|
Compound (chemistry)
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazenes
image:Dacarbazine.svg, 200px, Dacarbazine is a triazene used in the treatment of melanoma and Hodgkin's lymphoma. Triazenes are organic compounds that contain the functional group R1−N=N−NR2R3, where the R are each any of various types of substituent groups. Some medications and dyes are triazenes. Formally, the triazenes are related to the unstable chemical triazene, H2N−N=NH. Production Triazenes are prepared from the ''N''-coupling reaction between diazonium salts and primary or secondary amines. The coupling reactions are typically mild, using a base such as sodium acetate, sodium carbonate, or sodium bicarbonate. The diazonium reagents are themselves available starting from amines. For symmetrical triazenes derived from primary amines, partial diazotization gives a mixture of the original amine and its diazo derivative that then couple with each other. For example, 1,3-diphenyltriazene (PhN=N−NHPh) can be made from aniline in a one-pot reaction. For asymmetrical triaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company has two United States headquarters, in St. Louis and Burlington, MA and has operations in approximately 40 countries. In 2015, the multinational chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. It is headquartered in Burlington, Massachusetts, United States. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct Yellow 4
Direct Yellow 4 is a chemical compound with the formula . This is a direct dis-azo dye, a diamine derivative with separated azo groups. Due to its properties, value and strength, it is considered one of the most important dyes based on stilbene. It can be used as an acid-base indicator. In an alkaline environment, the yellow color of the dye deepens through orange to red at a pH of 6.4 → 8.0. Synthesis The compound can be obtained by coupling tetrazotized diaminostil-benesulfonic acid twice to phenol. Physical properties The compound forms orange powder. It is water-soluble, producing a golden brown color, and moderately soluble in ethanol, also yielding a golden brown shade. It is slightly soluble in acetone and soluble in fiber materials. When treated with concentrated sulfuric acid, it turns red to light purple; diluted sulfuric acid produces a purple color with blackish light purple precipitate. In nitric acid solution, it shows a dark brown color (not in all solutions).{{ci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonates
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: : An alternative is the condensation of a sulfonyl halid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Sodium Salts
Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product of decay, or is composed of organic compounds * Organic compound, a compound that contains carbon ** Organic chemistry, chemistry involving organic compounds Farming, certification and products * Organic farming, agriculture conducted according to certain standards, especially the use of stated methods of fertilization and pest control * Organic certification, accreditation process for producers of organically-farmed products * Organic horticulture, the science and art of growing fruits, vegetables, flowers, or ornamental plants by following the essential principles of organic agriculture * Organic products, "organics": ** Organic food, food produced from organic farming methods and often certified organic according to organic farming s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzothiazoles
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula . It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole. It has a sulfurous odor and meaty flavor. The three structural isomer, structural isomers of benzothizaole are 1,3-benzothiazole, 1,2-benzothiazole and 2,1-benzothiazole. Structure and reactivity Benzothiazoles consist of a 5-membered 1,3-thiazole ring Annulation, fused to a benzene ring. The nine atoms of the bicycle and the attached substituents are coplanar. The heterocyclic core of the molecule is readily substituted at the Methine group, methyne (CH) centre in the thiazole ring. Thiazole is electron-withdrawing. Synthesis and biosynthesis Benzothiazoles are typically prepared by treatment of 2-Aminothiophenol, 2-merc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Dyes
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to macromolecules, serving as a markers (or dyes, or tags, or reporters) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, such as fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |