|
Tin(IV) Fluoride
Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4. It is a white solid. As reflected by its melting point above 700 °C, the tetrafluoride differs significantly from the other tetrahalides of tin. Synthesis and reaction SnF4 can be prepared by the reaction of tin(IV) chloride with anhydrous hydrogen fluoride: :SnCl4 + 4HF → SnF4 + 4HCl When treated with alkali metal fluorides (e.g. KF), tin(IV) fluoride forms hexafluorostannates: : In K2SnF6, tin adopts an octahedral geometry. Otherwise, SnF4 behaves as a Lewis acid forming a variety of adducts with the formula L2·SnF4 and L·SnF4. Structure Unlike the heavier tin tetrahalides, which contain tetrahedrally coordinated tin, tin(IV) fluoride contains octahedrally coordinated tin. The octahedra share four corners. There are two terminal, unshared, fluorine atoms ''trans Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of". Used alone, trans may refer to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Prism (geometry), prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The body-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Chloride
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound of tin and chlorine with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as ''spiritus fumans libavii''. Preparation It is prepared from reaction of chlorine gas with tin at : : Structure Anhydrous tin(IV) chloride solidifies at −33 °C to give monoclinic crystals with the P21/c space group. It is isostructural with SnBr4. The molecules adopt near-perfect tetrahedral symmetry with average Sn–Cl distances of 227.9(3) pm. Reactions Tin(IV) chloride is well known as a Lewis acid. Thus it forms hydrates. The pentahydrate SnCl4·5H2O was formerly known as butter of tin. These hydrates consist of ''cis''- nCl4(H2O)2molecules together with varying amounts of water of crystallization. The additional water molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
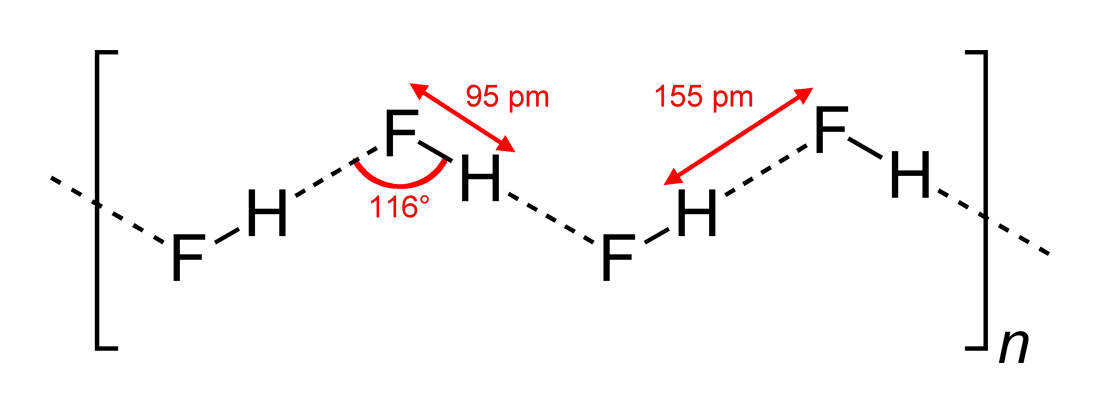
Fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The nome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannous Fluoride
Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin ', 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes. Oral health benefits Stannous fluoride is an alternative to sodium fluoride for the prevention of cavities ( tooth decay). It was first released commercially in 1956, in Crest toothpaste. It was discovered and developed by Joseph Muhler and William Nebergall. In recognition of their innovation, they were inducted into the Inventor's Hall of Fame. The fluoride in stannous fluoride helps to convert the calcium mineral hydroxyapatite in teeth into fluorapatite, which makes tooth enamel more resistant to bacteria-generated acid attacks. The calcium present in plaque and saliva reacts with fluoride to form calcium fluoride on the tooth surface; over time, this calcium fluoride dissolves to allow calcium and fluoride ions to interact with the tooth and form fluoride-containing ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Tetrafluoride
Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula Si F4. This colorless gas is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first prepared in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid, and later synthesized by John Davy in 1812. It is a tetrahedral molecule and is corrosive. Occurrence Volcanic plumes contain significant amounts of silicon tetrafluoride. Production can reach several tonnes per day. Some amounts are also emitted from spontaneous coal fires.Kruszewski, Ł., Fabiańska, M.J., Ciesielczuk, J., Segit, T., Orłowski, R., Motyliński, R., Moszumańska, I., Kusy, D. 2018 – First multi-tool exploration of a gas-condensate-pyrolysate system from the environment of burning coal mine heaps: An in situ FTIR and laboratory GC and PXRD study based on Upper Silesian materials. Science of the Total Environment, 640-641, 1044-1071; DOI: 10.1016/j.sci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrafluoride
Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (Carbon, CFluorine, F4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond. Bonding Because of the multiple carbon–fluorine bonds, and the high electronegativity of fluorine, the carbon in tetrafluoromethane has a significant positive partial charge which strengthens and shortens the four carbon–fluorine bonds by providing additional Ionic bond, ionic character. Carbon–fluorine bonds are the strongest single bonds in organic chemistry. Additionally, they strengthen as more carbon–fluorine bonds are added to the same carbon atom. In the one-carbon organofluorine compounds represented by molecules of fluorometh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Iodide
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula . This tetrahedral molecule crystallizes as a bright orange solid that dissolves readily in nonpolar solvents such as benzene. Preparation The compound is usually prepared by the reaction of tin and iodine: : Chemical properties The compound hydrolyses in water. In hydroiodic acid, it reacts to form a rare example of a hexaiodometallate (here hexaiodostannate(IV)): : SnI4 + 2 I− → nI6sup>2− Physical properties Tin(IV) iodide is an orange solid under standard conditions. It has a cubic crystal structure with the space group ''Pa'' (space group no. 205), the lattice parameter a = 1226 pm and eight formula units per unit cell. This corresponds approximately to a cubic close packing of iodine atoms in which 1/8 of all tetrahedral gaps are occupied by tin atoms. This leads to discrete tetrahedral SnI4 molecules. See also * Tin(II) iodide *Tin(IV) chloride Tin(IV) chloride, al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Bromide
Tin(IV) bromide is the chemical compound SnBr4. It is a colourless low melting solid. Structure SnBr4 crystallises in a monoclinic crystal system with molecular SnBr4 units that have distorted tetrahedral geometry. The mean Sn-Br bond length is 242.3 pm. Preparation SnBr4 can be prepared by reaction of the elements at standard temperature and pressure (STP): : + 2 → Reactions In aqueous solution SnBr4 dissolves to give a series of octahedral (six-ligated) bromo-aquo complexes. These include and ''cis''- and ''trans''-. SnBr4 forms 1:1 and 1:2 complexes with ligands. With trimethylphosphine both . Tin(IV) bromide undergoes redistribution with tin(IV) chloride as assessed by 119Sn NMR and Raman spectroscopy Raman spectroscopy () (named after physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Ra .... Equilibrium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane CH3)3Bis a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |