|
Solubility Equilibrium
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent ''solubility product'' which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios. Definitions A solubility equilibrium exists when a chemical compound in the solid state is in chemical equilibrium with a solution containing the compound. This type of equilibrium is an example of dynamic equilibrium in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution and precipitation are equal to one another. When equilibrium is established and the solid has not all disso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances transition between the reactants and products at equal rates, meaning there is no net change. Reactants and products are formed at such a rate that the concentration of neither changes. It is a particular example of a system in a steady state. In physics, concerning thermodynamics, a closed system is in thermodynamic equilibrium when reactions occur at such rates that the composition of the mixture does not change with time. Reactions do in fact occur, sometimes vigorously, but to such an extent that changes in composition cannot be observed. Equilibrium constants can be expressed in terms of the rate constants for reversible reactions. Examples In a new bottle of soda, the concentration of carbon dioxide in the liquid phase has a particular value. If half of the liquid is poured out and the bottle is sealed, carbon dioxide will leave the liquid phase at an ever-decreasing rate, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimension
In physics and mathematics, the dimension of a mathematical space (or object) is informally defined as the minimum number of coordinates needed to specify any point within it. Thus, a line has a dimension of one (1D) because only one coordinate is needed to specify a point on itfor example, the point at 5 on a number line. A surface, such as the boundary of a cylinder or sphere, has a dimension of two (2D) because two coordinates are needed to specify a point on itfor example, both a latitude and longitude are required to locate a point on the surface of a sphere. A two-dimensional Euclidean space is a two-dimensional space on the plane. The inside of a cube, a cylinder or a sphere is three-dimensional (3D) because three coordinates are needed to locate a point within these spaces. In classical mechanics, space and time are different categories and refer to absolute space and time. That conception of the world is a four-dimensional space but not the one that was f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gravimetric Analysis
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known. The four main types of this method of analysis are ''precipitation'', ''volatilization'', ''electro-analytical'' and ''miscellaneous physical method''. The methods involve changing the phase of the analyte to separate it in its pure form from the original mixture and are quantitative measurements. Precipitation method The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H2C2O4, is added to a measured, known volume of water. By adding a reagent, here ammoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Chloride
Silver chloride is a chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water (this behavior being reminiscent of the chlorides of Tl+ and Pb2+). Upon illumination or heating, silver chloride converts to silver (and chlorine), which is signaled by grey to black or purplish coloration to some samples. AgCl occurs naturally as a mineral chlorargyrite. Preparation Silver chloride is unusual in that, unlike most chloride salts, it has very low solubility. It is easily synthesized by metathesis: combining an aqueous solution of silver nitrate (which is soluble) with a soluble chloride salt, such as sodium chloride or cobalt(II) chloride. The silver chloride that forms will precipitate immediately. :AgNO3 + NaCl -> AgCl(v) + NaNO3 :2 AgNO3 + CoCl2 -> 2 AgCl(v) + Co(NO3)2 Structure and reactions The solid adopts the ''fcc'' NaCl structure, in which each Ag+ ion is surrounded by an octahedron of six chloride li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Common-ion Effect
The common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier's principle for the equilibrium reaction of the ionic association/dissociation. The effect is commonly seen as an effect on the solubility of salts and other weak electrolytes. Adding an additional amount of one of the ions of the salt generally leads to increased precipitation of the salt, which reduces the concentration of both ions of the salt until the solubility equilibrium is reached. The effect is based on the fact that both the original salt and the other added chemical have one ion in common with each other. Examples of the common-ion effect Dissociation of hydrogen sulfide in presence of hydrochloric acid Hydrogen sulfide (H2S) is a weak electrolyte. It is partially ionized when in aqueous solution, therefore there exists an equilibrium b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Atkins
Peter William Atkins (born 10 August 1940) is an English chemist and a Fellow of Lincoln College at the University of Oxford. He retired in 2007. He is a prolific writer of popular chemistry textbooks, including ''Physical Chemistry'', ''Inorganic Chemistry'', and ''Molecular Quantum Mechanics''. Atkins is also the author of a number of popular science books, including ''Atkins' Molecules'', ''Galileo's Finger: The Ten Great Ideas of Science'' and ''On Being''. Career Atkins left school ( Dr Challoner's Grammar School, Amersham) at fifteen and took a job at Monsanto as a laboratory assistant. He studied for A-levels by himself and gained a place, following a last-minute interview, at the University of Leicester. Atkins studied chemistry there, obtaining a BSc degree in chemistry, and a PhD degree in 1964 for research into electron spin resonance spectroscopy, and other aspects of theoretical chemistry. Atkins then took a postdoctoral position at UCLA as a Harkness Fellow o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kenneth Denbigh
Kenneth George Denbigh FRS (30 May 1911 – 23 January 2004) was an English chemical engineer and scientific philosopher. He wrote much on the issue of time in relation to thermodynamics. He was an associate of the Russian chemist Georgi Gladyshev. The University of Edinburgh named the Kenneth Denbigh Building at King's Buildings in his honour. They also offer a Kenneth Denbigh Scholarship to science students. ThSchool of Engineering of The University of Edinburghawards the Kenneth Denbigh Medal in support of his scientific legacy. The Medal has been first established in 2023 and awarded during th10th Heat Powered Cycles Conference Life He was born in Luton on 30 May 1911 the son of George Denbigh, manager of Brothertons Chemical Works in Wakefield. He attended the University of Leeds graduating with a BSc in 1932. He then undertook his doctorate under Robert Whytlaw-Gray gaining a PhD in 1934. He worked for Imperial Chemical Industries (ICI) until 1938 when obtained a post ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
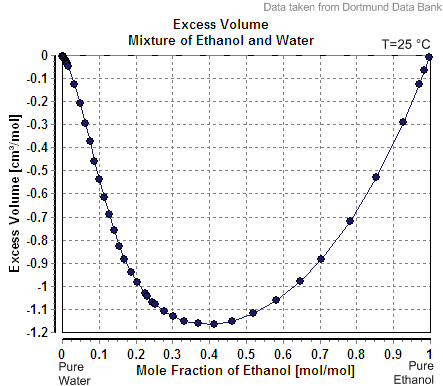
Partial Molar Enthalpy
In thermodynamics, a partial molar property is a quantity which describes the variation of an extensive property of a solution or mixture with changes in the molar composition of the mixture at constant temperature and pressure. It is the partial derivative of the extensive property with respect to the amount (number of moles) of the component of interest. Every extensive property of a mixture has a corresponding partial molar property. Definition The partial molar volume is broadly understood as the contribution that a component of a mixture makes to the overall volume of the solution. However, there is more to it than this: When one mole of water is added to a large volume of water at 25 °C, the volume increases by 18 cm3. The molar volume of pure water would thus be reported as 18 cm3 mol−1. However, addition of one mole of water to a large volume of pure ethanol results in an increase in volume of only 14 cm3. The reason that the increase is differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides. Forms *Anhydrous sodium sulfate, known as the rare mineral thenardite, used as a drying agent in organic synthesis. *Heptahydrate sodium sulfate, a very rare form. *Decahydrate sodium sulfate, known as the mineral mirabilite, widely used by chemical industry. It is also known as Glauber's salt. History The decahydrate of sodium sulfate is known as Glauber's salt after the Dutch/German chemist and apothecary Johann Rudolf Glauber (1604–1670), who discovered it in Austrian spring water in 1625. He name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen). The term ''exothermic'' was first coined by 19th-century French chemist Marcellin Berthelot. The opposite of an exothermic process is an endothermic process, one that absorbs energy usually in the form of heat. The concept is frequently applied in the physical sciences to chemical reactions where chemical bond energy is converted to thermal energy (heat). Two types of chemical reactions Exothermic and endothermic describe two types of chemical reactions or systems found in nature, as follows: Exothermic After an exothermic reaction, more energy has been released to the surroundings than was absorbed to initiate and maintain the reaction. An example would be t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recrystallization (chemistry)
In chemistry, recrystallization is a technique used to purify chemicals. By dissolving a mixture of a compound and impurities in an appropriate solvent, either the desired compound or impurities can be removed from the solution, leaving the other behind. It is named for the crystals often formed when the compound precipitates out. Alternatively, ''recrystallization'' can refer to the natural growth of larger ice crystals at the expense of smaller ones. Chemistry In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted (see Separation process), recrystallization being one of them. There are also different recrystallization techniques that can be used such as: Single-solvent recrystallization Typically, the mixture of "compound A" and "impurity B" is dissolved in the smallest amount of hot so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothermic Reaction
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. 617. In such a process, a closed system usually absorbs thermal energy from its surroundings, which is heat transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. It may be a chemical process, such as dissolving ammonium nitrate () in water (), or a physical process, such as the melting of ice cubes. The term was coined by 19th-century French chemist Marcellin Berthelot. The opposite of an endothermic process is an exothermic process, one that releases or "gives out" energy, usually in the form of heat and sometimes as electrical energy. Thus in each term (endothermic and exothermic) the prefix refers to where heat (or electric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |