|
Sodium Minerals
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals includin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity". Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, William Nicholson and Anthony Carlisle sought to further ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthocl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Na+/K+-ATPase
NA, N.A., Na, nA or n/a may refer to: Chemistry and physics * Sodium, symbol Na, a chemical element * Avogadro constant (''N''A) * Nucleophilic addition, a type of reaction in organic chemistry * Numerical aperture, a number that characterizes a range of angles in an optical system * nA, the symbol for nanoampere * Naturally aspirated engine Biology and medicine * Na (tree) or ''Mesua ferrea'', a species of tree native to Sri Lanka * Neuroacanthocytosis, a neurological condition * ''Nomina Anatomica'', a former international standard for human anatomical nomenclature * Noradrenaline, a hormone * Nucleic acid analogue, compounds analogous to naturally occurring RNA and DNA Places Current * Namibia (ISO country code) * Naples (car number plate code: NA), Italy * North America, a continent * North Africa, a subcontinent Historical * Netherlands Antilles (former international vehicle registration code: NA) * Na (Chinese state), a small state of the Chinese Zhou dynasty from the 11th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypovolemia
Hypovolemia, also known as volume depletion or volume contraction, is a state of abnormally low extracellular fluid in the body. This may be due to either a loss of both salt and water or a decrease in blood volume. Hypovolemia refers to the loss of extracellular fluid and should not be confused with dehydration. Hypovolemia is caused by a variety of events, but these can be simplified into two categories: those that are associated with kidney function and those that are not. The signs and symptoms of hypovolemia worsen as the amount of fluid lost increases. Immediately or shortly after mild fluid loss (from blood donation, diarrhea, vomiting, bleeding from trauma, etc.), one may experience headache, fatigue, weakness, dizziness, or thirst. Untreated hypovolemia or excessive and rapid losses of volume may lead to hypovolemic shock. Signs and symptoms of hypovolemic shock include increased heart rate, low blood pressure, pale or cold skin, and altered mental status. When these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tonicity
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution. Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''.A hypotonic s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypernatremia
Hypernatremia, also spelled hypernatraemia, is a high concentration of sodium in the blood. Early symptoms may include a strong feeling of thirst, weakness, nausea, and loss of appetite. Severe symptoms include confusion, muscle twitching, and bleeding in or around the brain. Normal serum sodium levels are 135–145 mmol/L (135–145 mEq/L). Hypernatremia is generally defined as a serum sodium level of more than 145 mmol/L. Severe symptoms typically only occur when levels are above 160 mmol/L. Hypernatremia is typically classified by a person's fluid status into low volume, normal volume, and high volume. Low volume hypernatremia can occur from sweating, vomiting, diarrhea, diuretic medication, or kidney disease. Normal volume hypernatremia can be due to fever, extreme thirst, prolonged increased breath rate, diabetes insipidus, and from lithium among other causes. High volume hypernatremia can be due to hyperaldosteronism, excessive administration of intrav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until equilibrium is attained. Theory and measurement Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expressed in the following equation: :\Pi = icRT where \Pi is osmotic p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
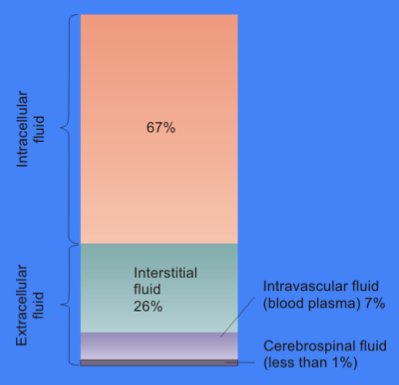
Extracellular Fluid
In cell biology, extracellular fluid (ECF) denotes all body fluid outside the cells of any multicellular organism. Total body water in healthy adults is about 60% (range 45 to 75%) of total body weight; women and the obese typically have a lower percentage than lean men. Extracellular fluid makes up about one-third of body fluid, the remaining two-thirds is intracellular fluid within cells. The main component of the extracellular fluid is the interstitial fluid that surrounds cells. Extracellular fluid is the internal environment of all multicellular animals, and in those animals with a blood circulatory system, a proportion of this fluid is blood plasma. Plasma and interstitial fluid are the two components that make up at least 97% of the ECF. Lymph makes up a small percentage of the interstitial fluid. The remaining small portion of the ECF includes the transcellular fluid (about 2.5%). The ECF can also be seen as having two components – plasma and lymph as a delivery s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dietary Mineral
In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight (oxygen, hydrogen, carbon, and nitrogen), are usually not included in lists of major nutrient minerals (nitrogen is considered a "mineral" for plants, as it often is included in fertilizers). These four elements compose about 96% of the weight of the human body, and major minerals (macrominerals) and minor minerals (also called trace elements) compose the remainder. Nutrient minerals, being elements, cannot be synthesized biochemically by living organisms. Plants get minerals from soil. Most of the minerals in a human diet come from eating plants and animals or from drinking water. As a group, ''minerals'' are one of the four groups of essential nutrients, the others of which are vitamins, essential fatty acids, and essential amino acids. The five major mineral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
De-ice
Deicing is the process of removing snow, ice or frost from a surface. Anti-icing is the application of chemicals that not only deice but also remain on a surface and continue to delay the reformation of ice for a certain period of time, or prevent adhesion of ice to make mechanical removal easier. Deicing can be accomplished by mechanical methods (scraping, pushing); through the application of heat; by use of dry or liquid chemicals designed to lower the freezing point of water (various salts or brines, alcohols, glycols); or by a combination of these different techniques. Application areas Roadways In 2013, an estimated 14 million tons of salt were used for deicing roads in North America. Deicing of roads has traditionally been done with salt, spread by snowplows or dump trucks designed to spread it, often mixed with sand and gravel, on slick roads. Sodium chloride (rock salt) is normally used, as it is inexpensive and readily available in large quantities. However, sinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edible Salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantities in seawater. The open ocean has about of solids per liter of sea water, a salinity of 3.5%. Salt is essential for life in general, and saltiness is one of the basic human tastes. Salt is one of the oldest and most ubiquitous food seasonings, and is known to uniformly improve the taste perception of food, including otherwise unpalatable food. Salting, brining, and pickling are also ancient and important methods of food preservation. Some of the earliest evidence of salt processing dates to around 6,000 BC, when people living in the area of present-day Romania boiled spring water to extract salts; a salt-works in China dates to approximately the same period. Salt was also prized by the ancient Hebrews, Greeks, Romans, Byzantine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

%2C_black_and_white.png)