|
Syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII (in Germany alone, half a million cars were built or rebuilt to run on wood gas). Production Syngas is produced by steam reforming or partial oxidation of natural gas or liquid hydrocarbons, or coal gasification. : : : Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of energy: : In principle, but rarely in practice, biomass and related hydrocarbon feedstocks could be used to generate biogas and biochar in waste-to-energy gasification facilities. The gas generated (mostly methane and carbon dioxide) is sometimes described as ''syng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock. An advantage of gasification is that syngas can be more efficient than direct combustion of the original feedstock material because it can be combusted at higher temperatures so that the thermodynamic upper limi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen. Coal is a type of fossil fuel, formed when dead plant matter decays into peat which is converted into coal by the heat and pressure of deep burial over millions of years. Vast deposits of coal originate in former wetlands called coal forests that covered much of the Earth's tropical land areas during the late Carboniferous (Pennsylvanian (geology), Pennsylvanian) and Permian times. Coal is used primarily as a fuel. While coal has been known and used for thousands of years, its usage was limited until the Industrial Revolution. With the invention of the steam engine, coal consumption increased. In 2020, coal supplied about a quarter of the world's primary energy and over a third of its Electricity generation, electricity. Some iron and steel-maki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochar
Biochar is a form of charcoal, sometimes modified, that is intended for organic use, as in soil. It is the lightweight black remnants remaining after the pyrolysis of biomass, consisting of carbon and ashes. Despite its name, biochar is sterile immediately after production and only gains biological life following assisted or incidental exposure to biota. Biochar is defined by the International Biochar Initiative as the "solid material obtained from the thermochemical conversion of biomass in an oxygen-limited environment". Biochar is mainly used in soils to increase soil aeration, reduce soil emissions of greenhouse gases, reduce nutrient leaching, reduce soil acidity, and potentially increase the water content of coarse soils. Biochar application may increase soil fertility and agricultural productivity. However, when applied excessively or made from feedstock unsuitable for the soil type, biochar soil amendments also have the potential for negative effects, including harm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly, natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of Ammonia production, ammonia or methanol. The reaction is represented by this equilibrium: :CH4 + H2O CO + 3 H2 The reaction is strongly endothermic (Δ''H''SR = 206 kJ/mol). Hydrogen produced by steam reforming is termed grey hydrogen, 'grey' hydrogen when the waste carbon dioxide is released to the atmosphere and blue hydrogen, 'blue' hydrogen when the carbon dioxide is (mostly) captured and stored geologically—see carbon capture and storage. Zero carbon green hydrogen, 'green' hydrogen is produced by Thermochemical cycle, thermochemical water splitting, using solar thermal, low- or zero-carbon electricity or waste heat, or electrolysis, using low- or zero-carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water-gas Shift Reaction
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer okewith air and gasifying it with steam". The caloric yield of the fuel produced by this method is about 10% of the yield from a modern syngas plant. The coke needed to produce water gas also costs significantly more than the precursors for syngas (mainly methane from natural gas), making water gas technology an even less attractive business proposition. Production Synthesis gas is made by passing steam over a red-hot carbon fuel such as coke: : (Δ''H'' = +131 kJ/mol) The reaction is endothermic, so the fuel must be continually re-heated to maintain the reaction. To do this, an air stream, which alternates with the vapor stream, is introduced to combust some of the carbon: : (Δ''H'' = −393 kJ/mol) Theoretically, to make 6 L of water gas, 5 L of air is required. Alternatively, to prevent contamination with nitrogen, energy can be p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waste-to-energy
Waste-to-energy (WtE) or energy-from-waste (EfW) refers to a series of processes designed to convert waste materials into usable forms of energy, typically electricity or heat. As a form of energy recovery, WtE plays a crucial role in both waste management and sustainable energy production by reducing the volume of waste in landfills and providing an alternative energy source. The most common method of WtE is direct combustion of waste to produce heat, which can then be used to generate electricity via steam turbines. This method is widely employed in many countries and offers a dual benefit: it disposes of waste while generating energy, making it an efficient process for both waste reduction and energy production. In addition to combustion, other WtE technologies focus on converting waste into fuel sources. For example, gasification and pyrolysis are processes that thermochemically decompose organic materials in the absence of oxygen to produce syngas, a synthetic gas prima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Reactor
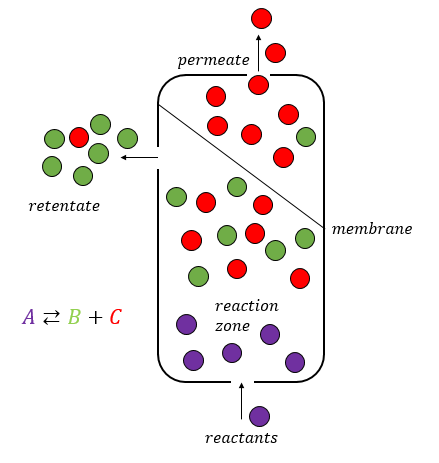
A membrane reactor is a physical device that combines a chemical conversion process with a membrane separation process to add reactants or remove products of the reaction. Chemical reactors making use of membranes are usually referred to as membrane reactors. The membrane can be used for different tasks: * Separation ** Selective extraction of products ** Retention of the catalyst * Distribution/dosing of a reactant * Catalyst support (often combined with distribution of reactants) Membrane reactors are an example for the combination of two unit operations in one step, e.g., membrane filtration with the chemical reaction. The integration of reaction section with selective extraction of a reactant allows an enhancement of the conversions compared to the equilibrium value. This characteristic makes membrane reactors suitable to perform equilibrium-limited endothermic reactions. Benefits and critical issues Selective membranes inside the reactor lead to several benefits: reacto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an Organic chemistry, organic Organic compound, compound, and among the simplest of organic compounds. Methane is also a hydrocarbon. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the Atmosphere of Earth, atmosphere, it is known as atmospheric methane. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Oxidation
Partial oxidation (POX) is a type of chemical reaction. It occurs when a substoichiometric fuel-air mixture is partially combusted in a reformer, creating a hydrogen-rich syngas which can then be put to further use, for example in a fuel cell. A distinction is made between ''thermal partial oxidation'' (TPOX) and ''catalytic partial oxidation'' (CPOX). Principle Partial oxidation is a technically mature process in which natural gas or a heavy hydrocarbon fuel (heating oil) is mixed with a limited amount of oxygen in an exothermic process. * General reaction: + \frac\mathit -> \mathit + \frac\mathitH2 * Idealized reaction for heating oil: + 6O2 -> + 12H2 * Idealized reaction for coal: + 12O2 -> + 6H2 The formulas given for coal and heating oil show only a typical representative of these complex fuels. Water may be added to lower the combustion temperature and reduce soot formation. Yields are below stoichiometric due to some fuel being fully combusted to carbon dioxide and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |